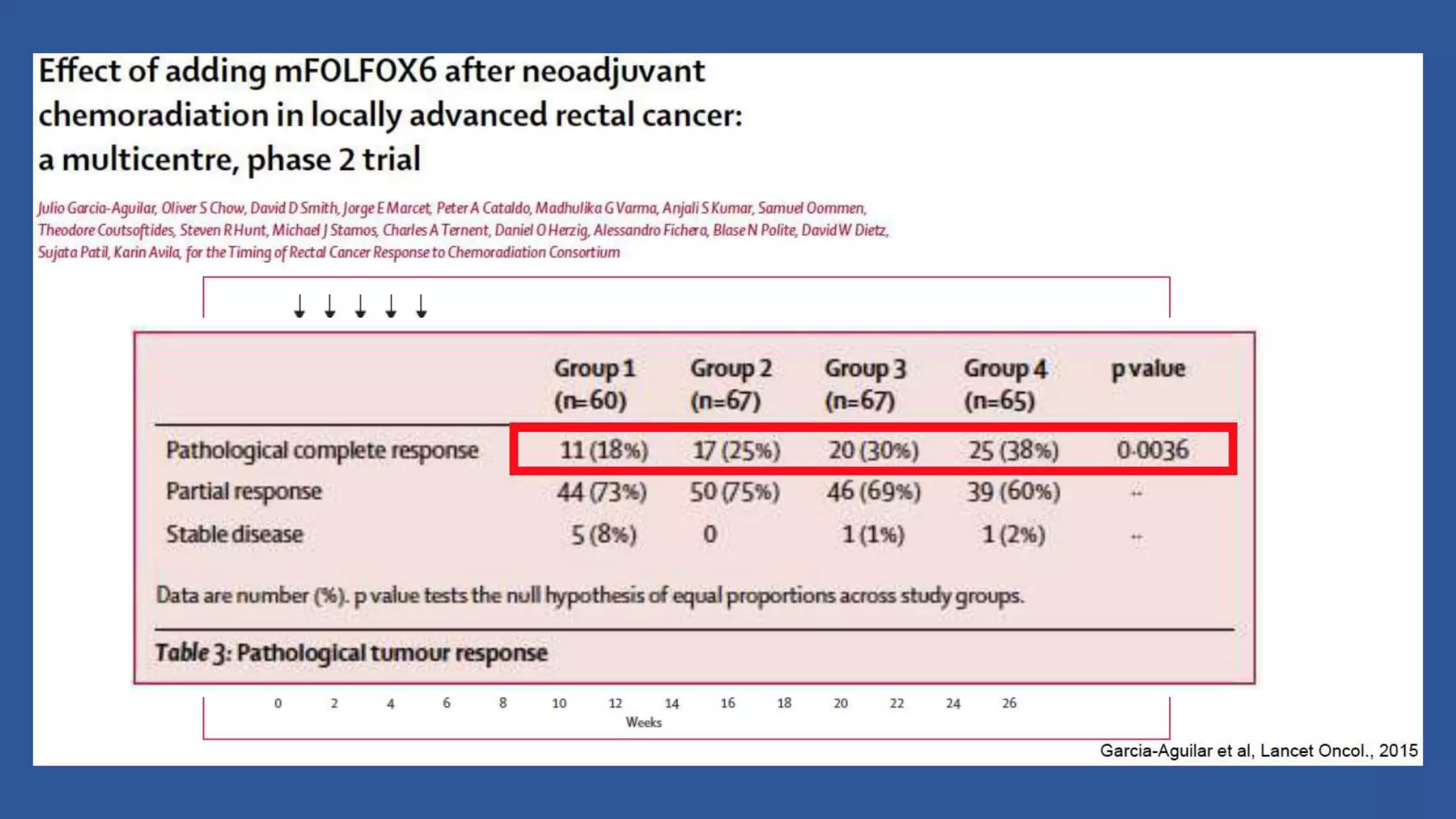
1) Pre-operative chemoradiotherapy remains the standard of care for stage 2-3 rectal cancer as it reduces local recurrence rates and allows for sphincter preservation.
2) For selected low-risk patients, de-intensified treatment with less surgery or radiation can be considered as local recurrence rates have reached low levels with current regimens.
3) High-risk patients still require trimodality treatment with chemotherapy, radiation, and surgery.
4) Biomarkers or functional imaging may help further select appropriate patients for de-intensified treatment. Distant metastases remain problematic and more effective systemic therapies are still needed.