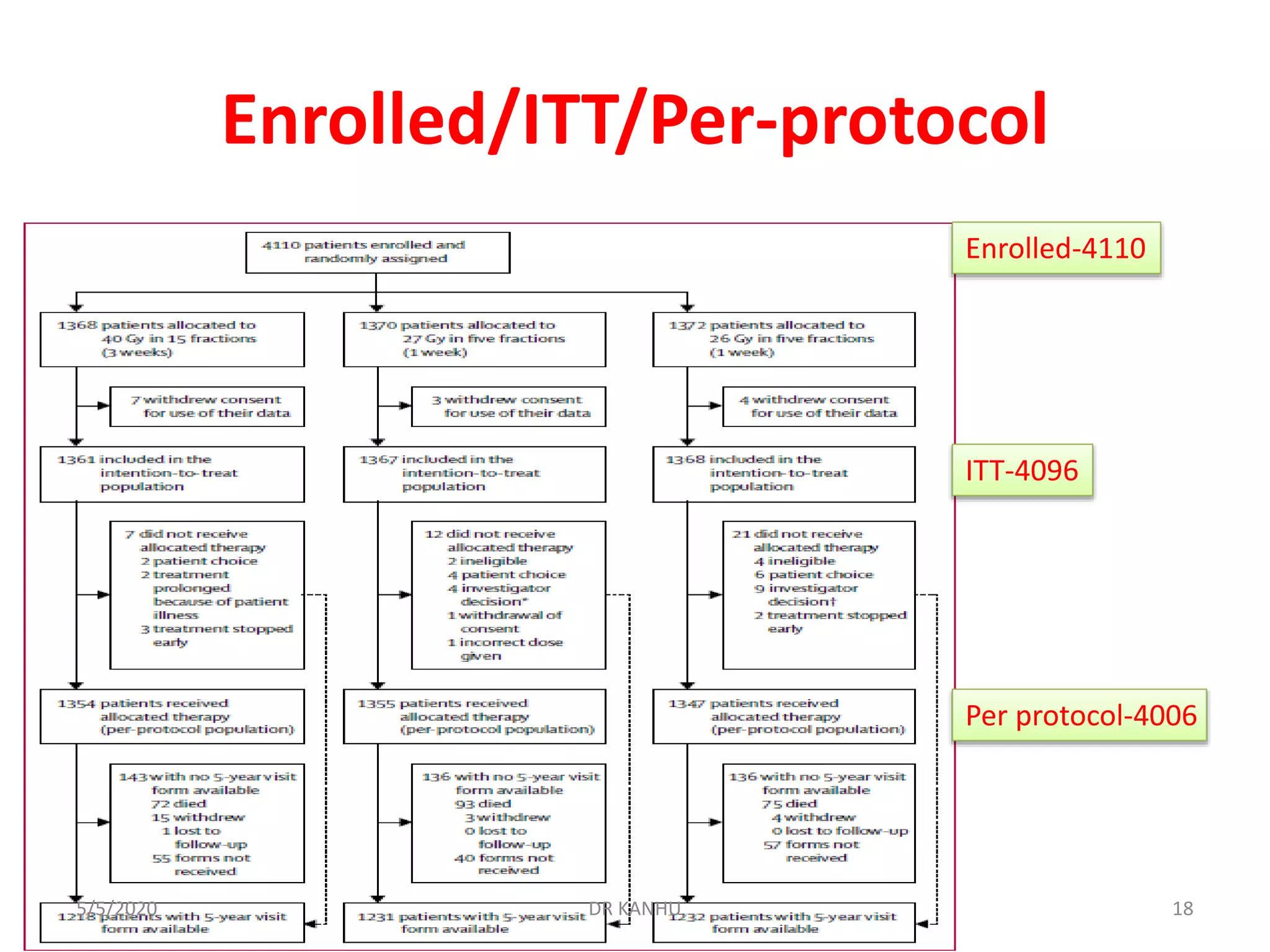
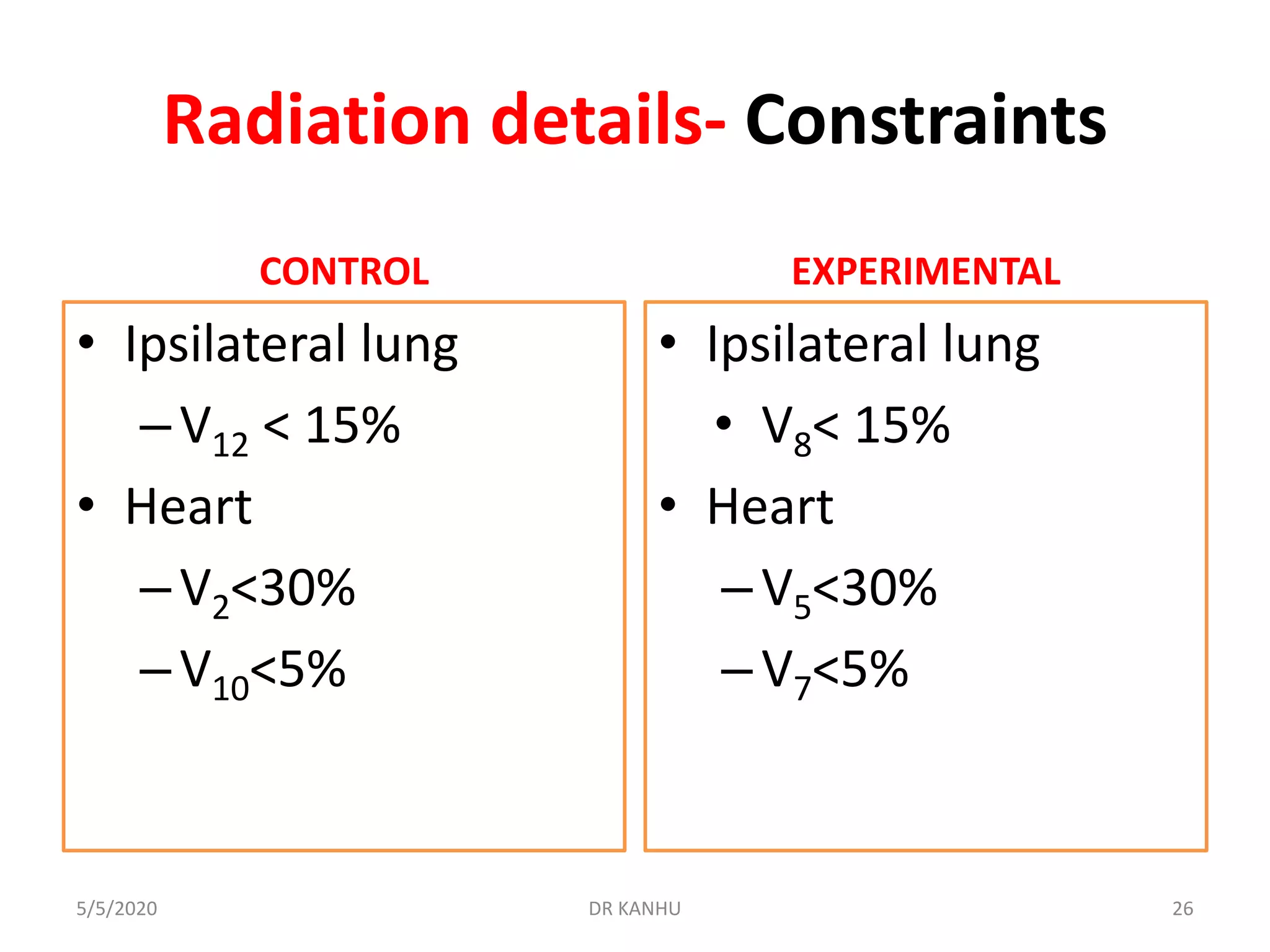
The document discusses the FAST Forward study, a phase III randomized trial comparing different fractionation schedules for adjuvant radiotherapy in breast cancer. The study involved 4096 patients randomized to receive either 40 Gy in 15 fractions, 27 Gy in 5 fractions, or 26 Gy in 5 fractions. At median follow-up of 71.5 months, there was no significant difference in ipsilateral breast tumor recurrence between groups. However, 27 Gy in 5 fractions was associated with significantly increased risk of moderate or marked late normal tissue effects compared to 40 Gy, based on clinical, patient-reported, and photographic assessments. Estimated α/β ratios were 3.7 Gy for tumor control and 1.7 Gy for normal tissue toxicity.















![Exclusions
• Aged ≥65 years, pT1, grade 1 or 2, [ER] pos.,
HER2 negative, pN0, M0
• Amendment on Feb 15, 2013
165/5/2020 DR KANHU](https://image.slidesharecdn.com/firstforward-200505111843/75/FAST-Forward-Trial-breast-cancer-16-2048.jpg)









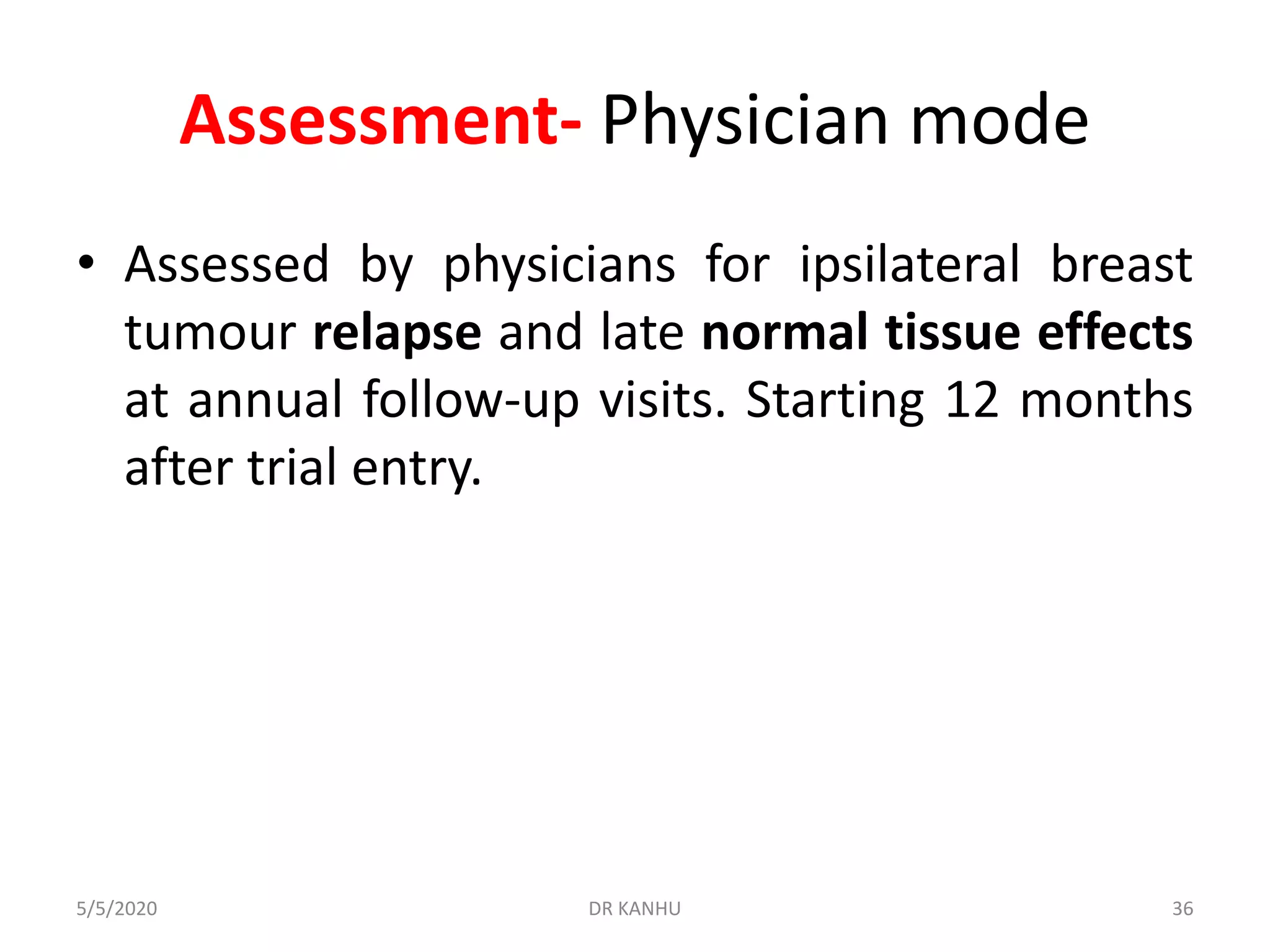
![OUTCOME end points
• Primary endpoint was ipsilateral breast tumor relapse
– Defined as invasive carcinoma or ductal carcinoma in situ
presenting anywhere in the ipsilateral breast parenchyma
or overlying skin or post-mastectomy chest wall, whether
considered local recurrence or new primary tumour.
• Secondary endpoints
– late normal tissue effects assessed by [PPP] physicians,
patients, and from photographs,
– Other disease-related and survival outcomes (loco regional
relapse, distant relapse, disease free survival, and overall
survival
295/5/2020 DR KANHU](https://image.slidesharecdn.com/firstforward-200505111843/75/FAST-Forward-Trial-breast-cancer-29-2048.jpg)


![Statistical analysis
• The primary endpoint was ipsilateral breast tumour
relapse; assuming a 2% 5-year incidence for 40 Gy,
non-inferiority was predefined as ≤1・6% excess for
5# schedules (critical hazard ratio [HR] of 1・81)
• Normal tissue effects were assessed by[PPP]
physicians, patients, and from photographs
• The database snapshot was taken on Nov 22, 2019;
Stata, version 15 (StataCorp)
335/5/2020 DR KANHU](https://image.slidesharecdn.com/firstforward-200505111843/75/FAST-Forward-Trial-breast-cancer-33-2048.jpg)













![Normal tissue effects
Photographic assessment
• 1737 patients
• At 2 years, mild or marked change in photographic breast appearance was
reported in
– 35/411 (8・5%) 40 Gy,
– 67/429 (15・6%) for 27 Gy
– 46/427 (10・8%) for 26 Gy;
• At 5 years were
– 34 (12・0%) of 283 for 40 Gy,
– 83 (26・9%) of 308 for 27 Gy
– 37 (13・0%) of 284 for 26 Gy Modeling
• 2-year and 5-year together
– 27Gy vs 40 Gy (OR 2・29 [95% CI 1・60 to 3・27], p<0・0001),
– 26Gy vs 40 Gy (OR 1・26 [0・85 to 1・86], p=0・24
– 26 Gy had a significantly lower risk of change in photographic breast
appearance than 27 Gy (p=0・0006).
– 27 Gy had a significantly increased risk of mild or marked change in breast
appearance
485/5/2020 DR KANHU](https://image.slidesharecdn.com/firstforward-200505111843/75/FAST-Forward-Trial-breast-cancer-48-2048.jpg)














