The document proposes fabricating solar cells on the lunar surface using in-situ resources to provide power for lunar bases. Key points include:
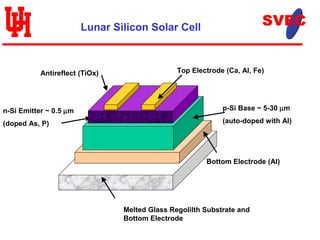
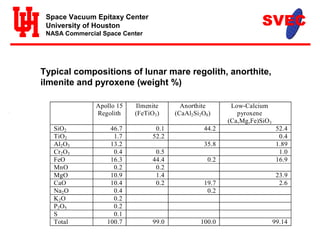
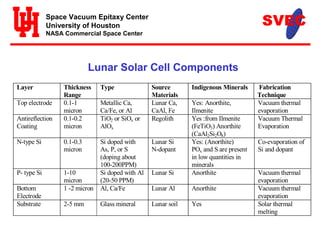
- Silicon, iron, titanium oxide, and other elements required for solar cells are found in lunar regolith. Vacuum evaporation would deposit thin film solar cells on melted glass substrates from regolith.
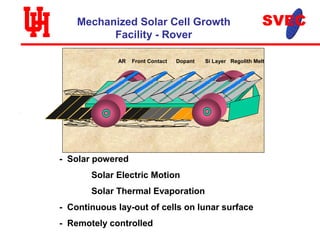
- A mechanized rover would continuously lay out and interconnect solar cells across the lunar surface, using solar heating and evaporation to process regolith and fabricate the cells.
- Initial objectives are to evaluate fabrication processes and raw material extraction, model the design's costs and benefits, and define tasks for further developing the concept to provide industrial-scale lunar power.