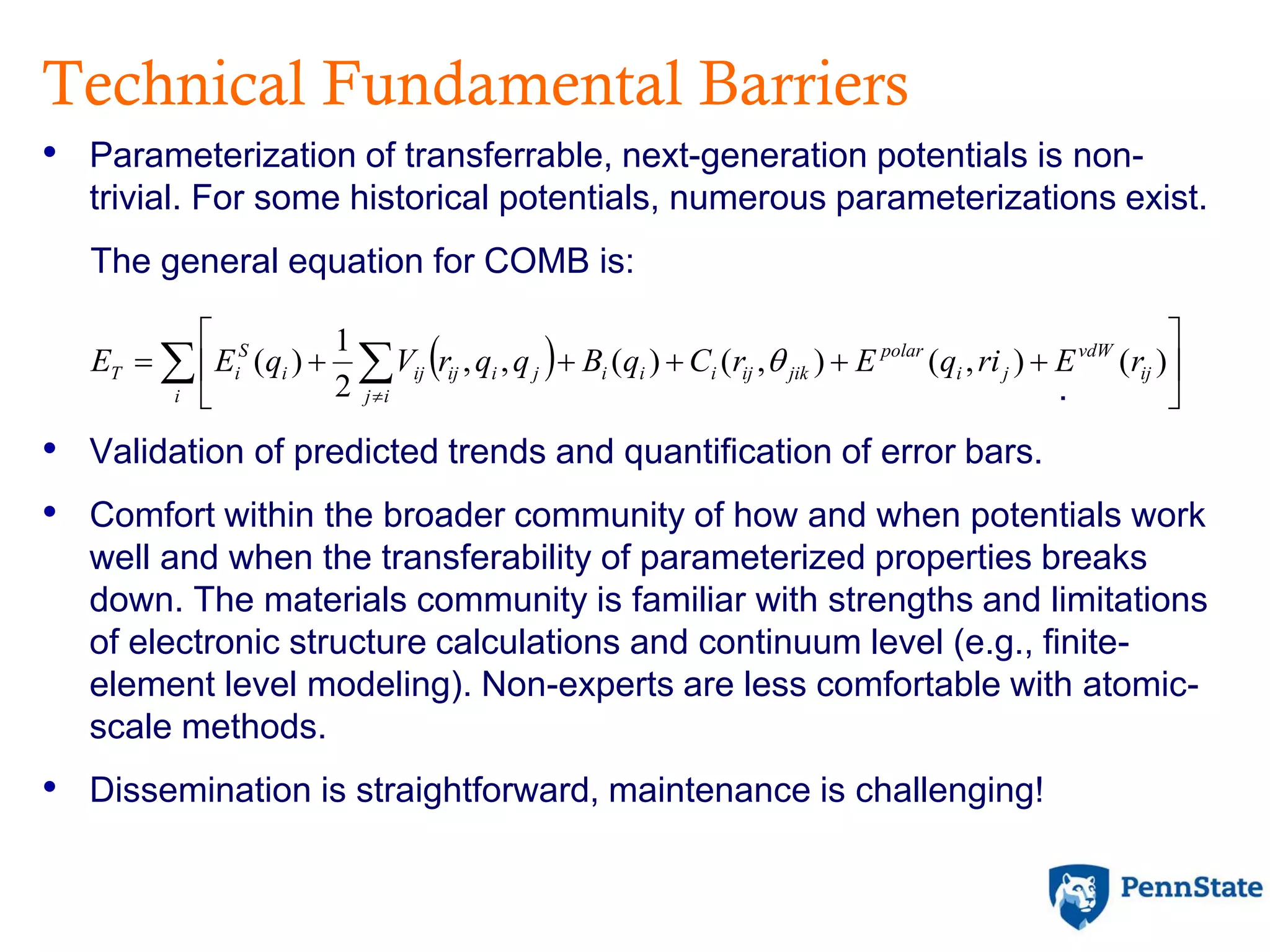
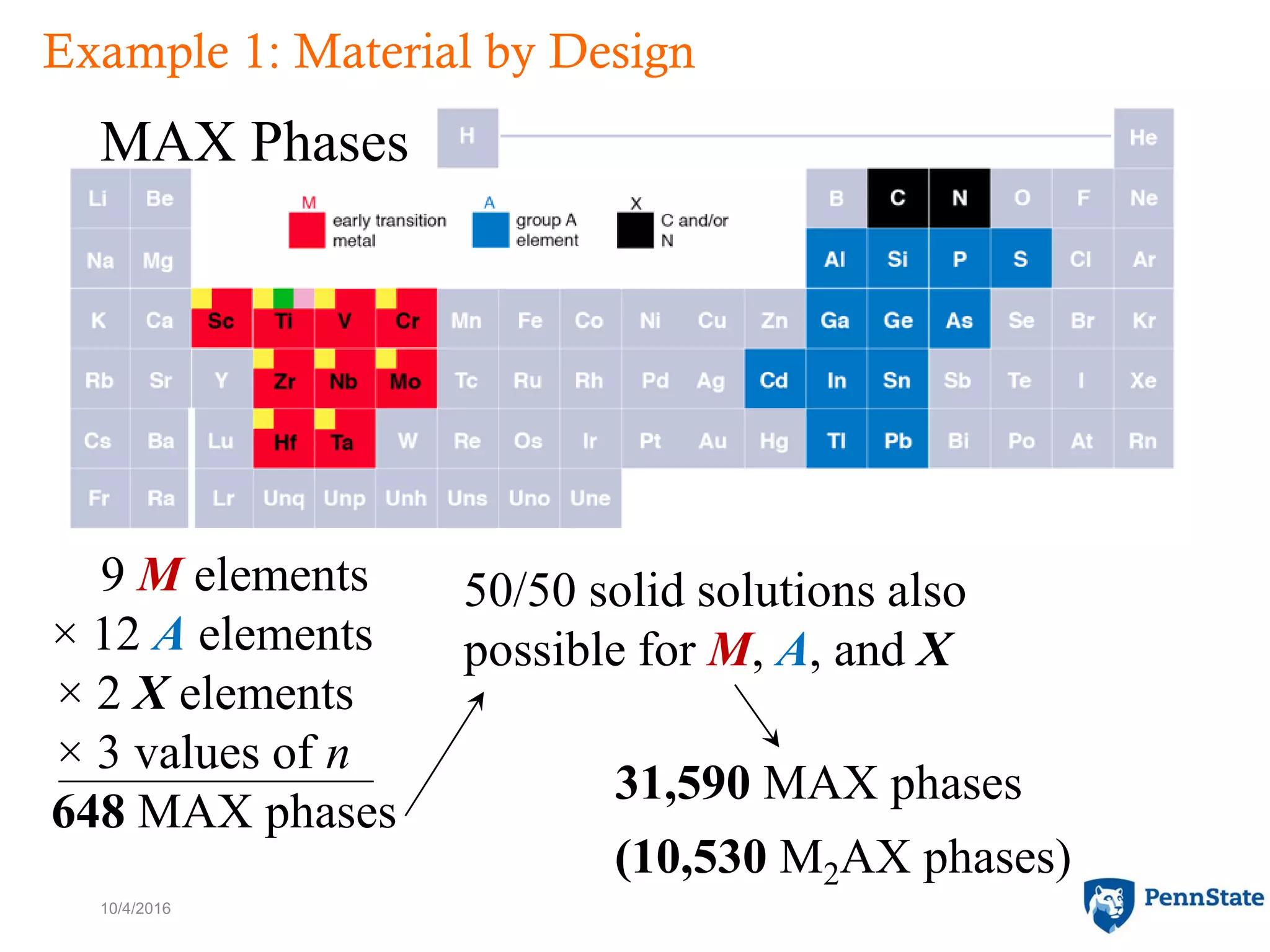
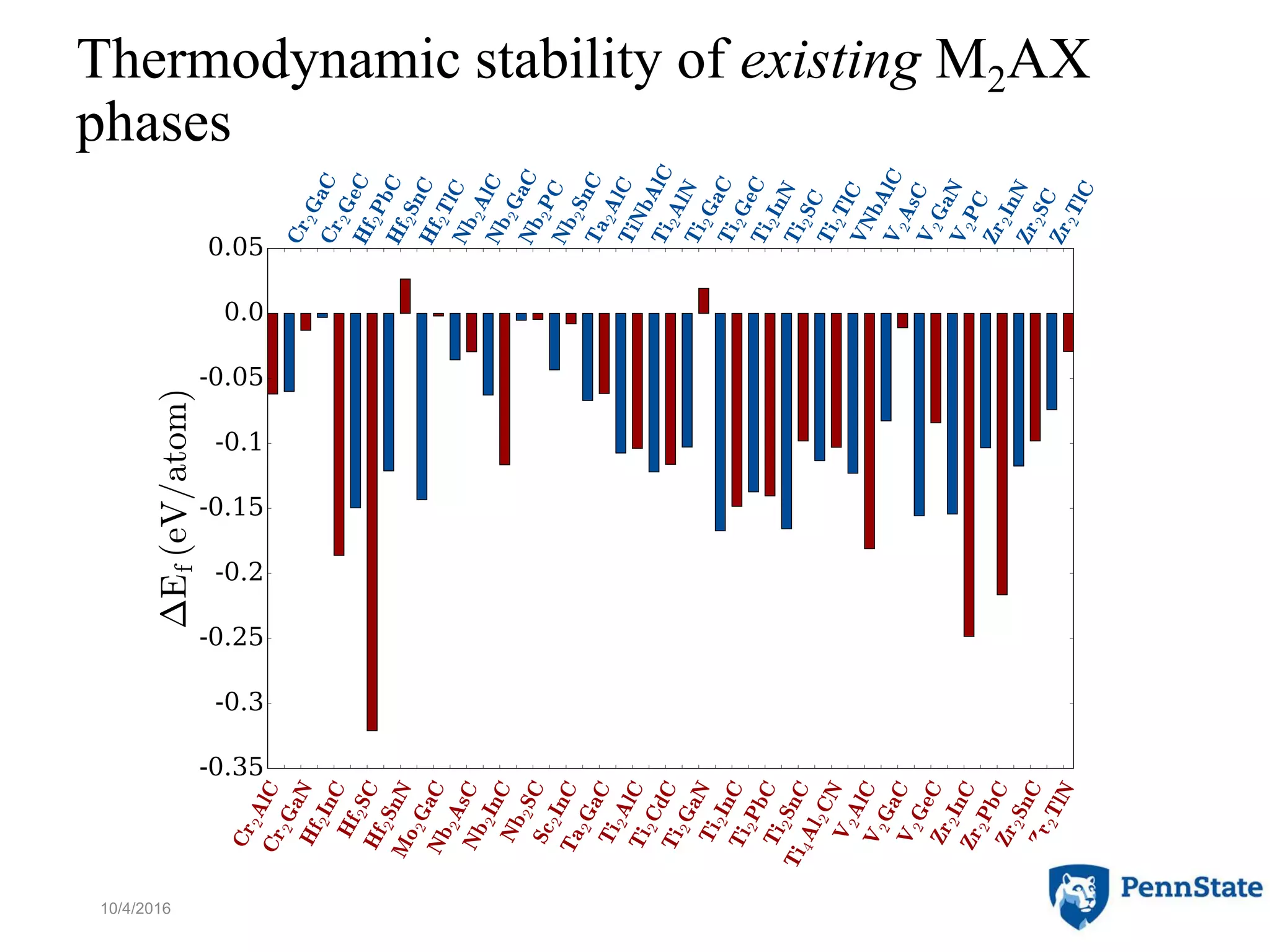
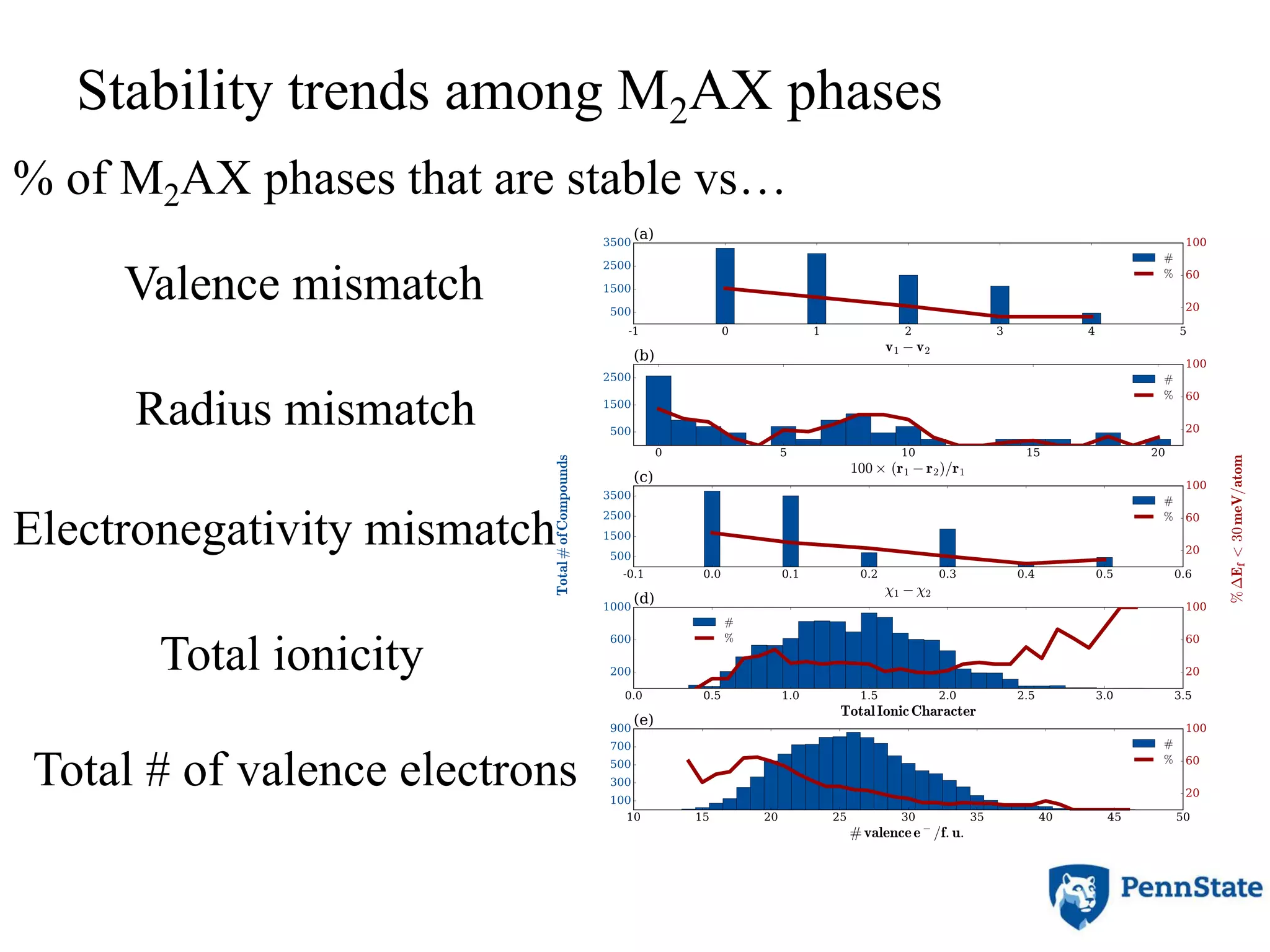
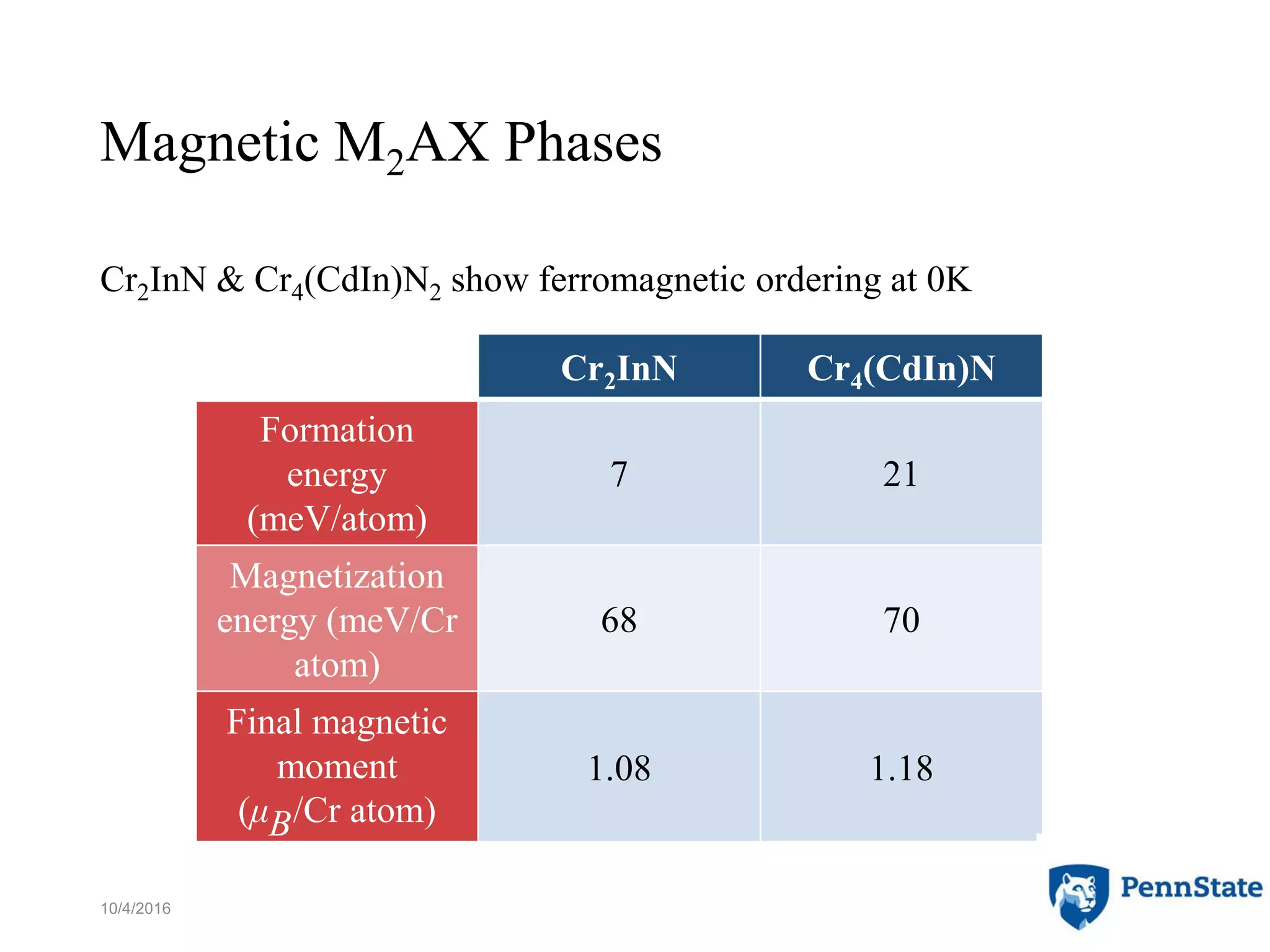
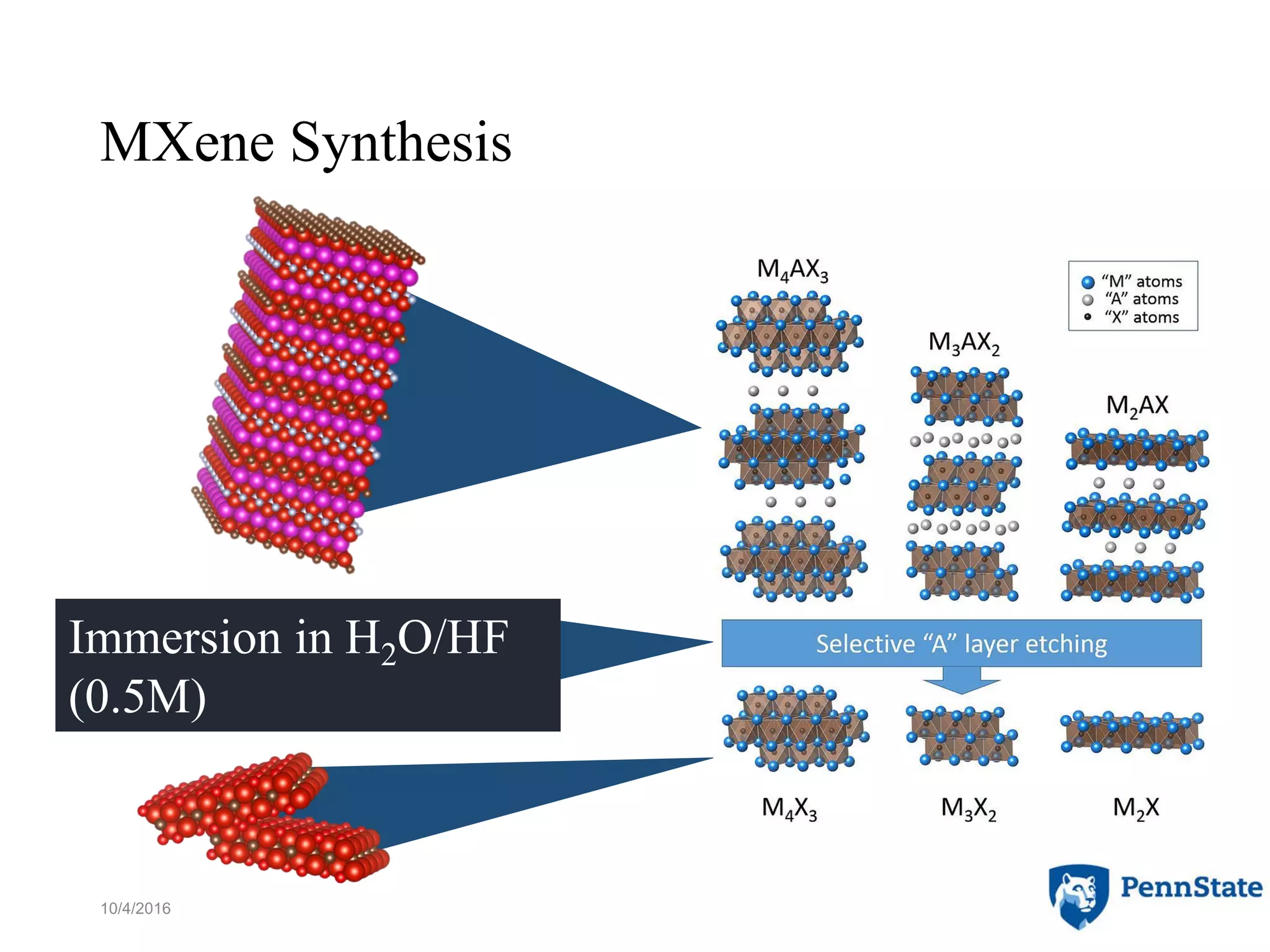
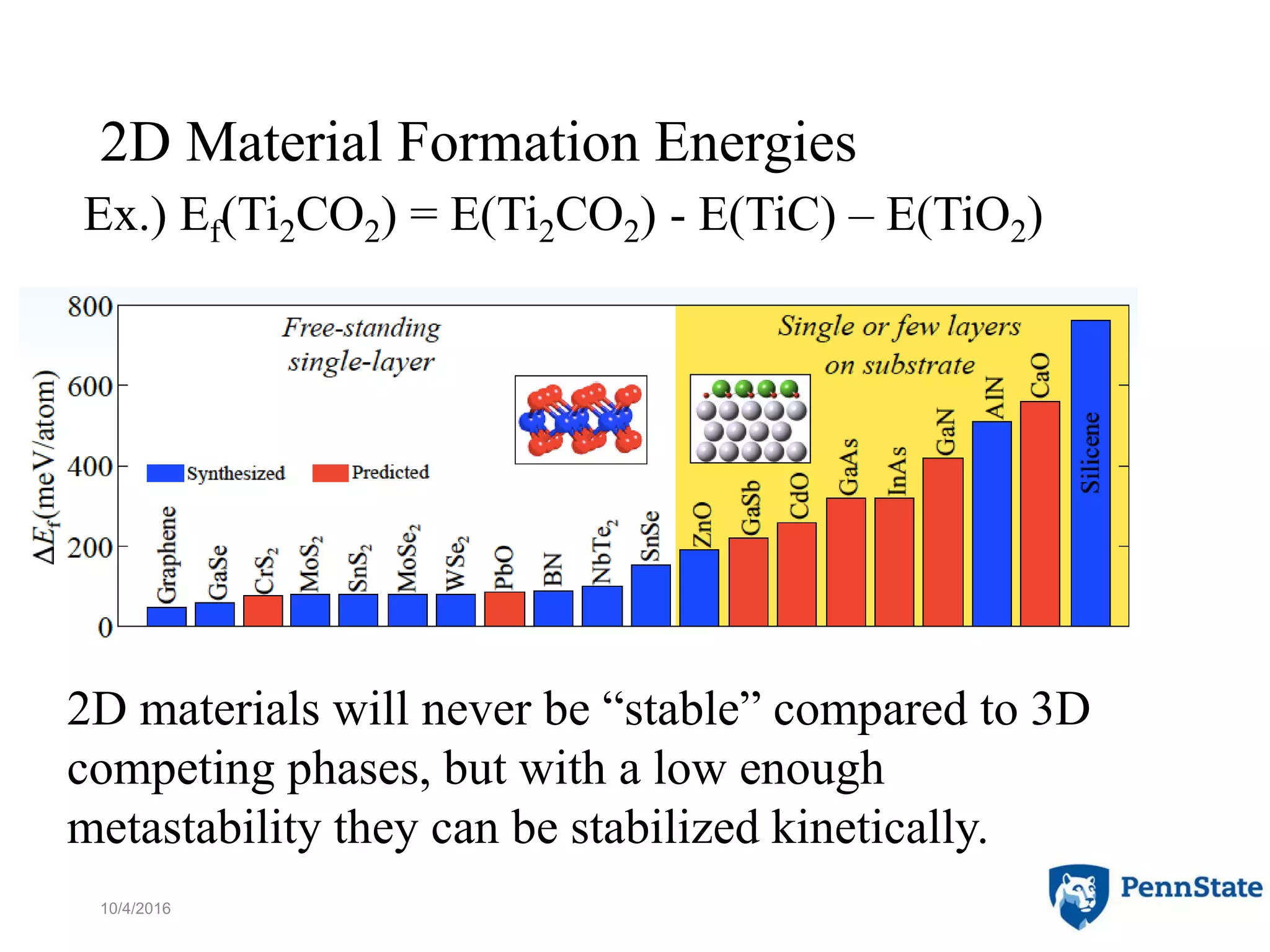
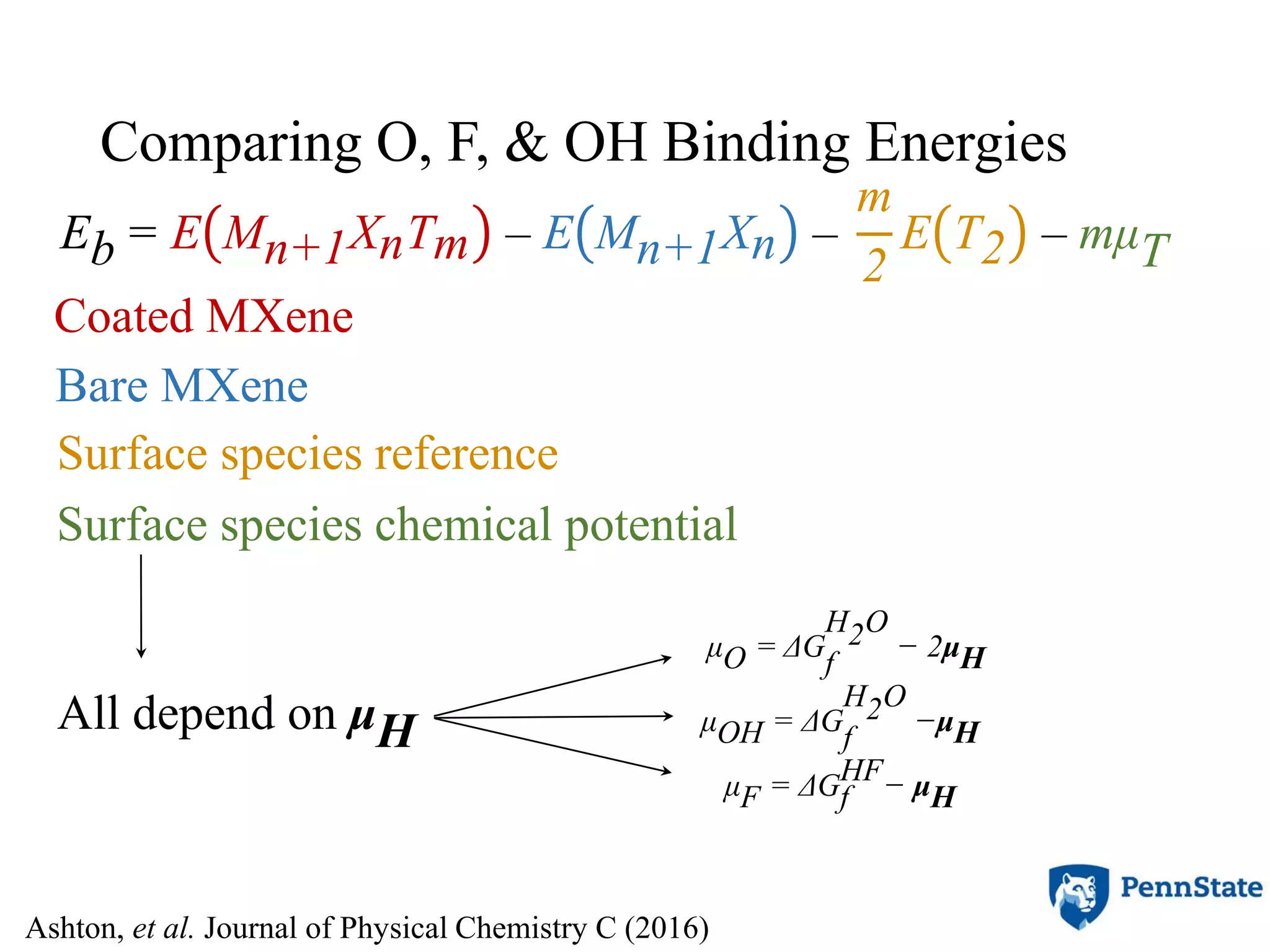
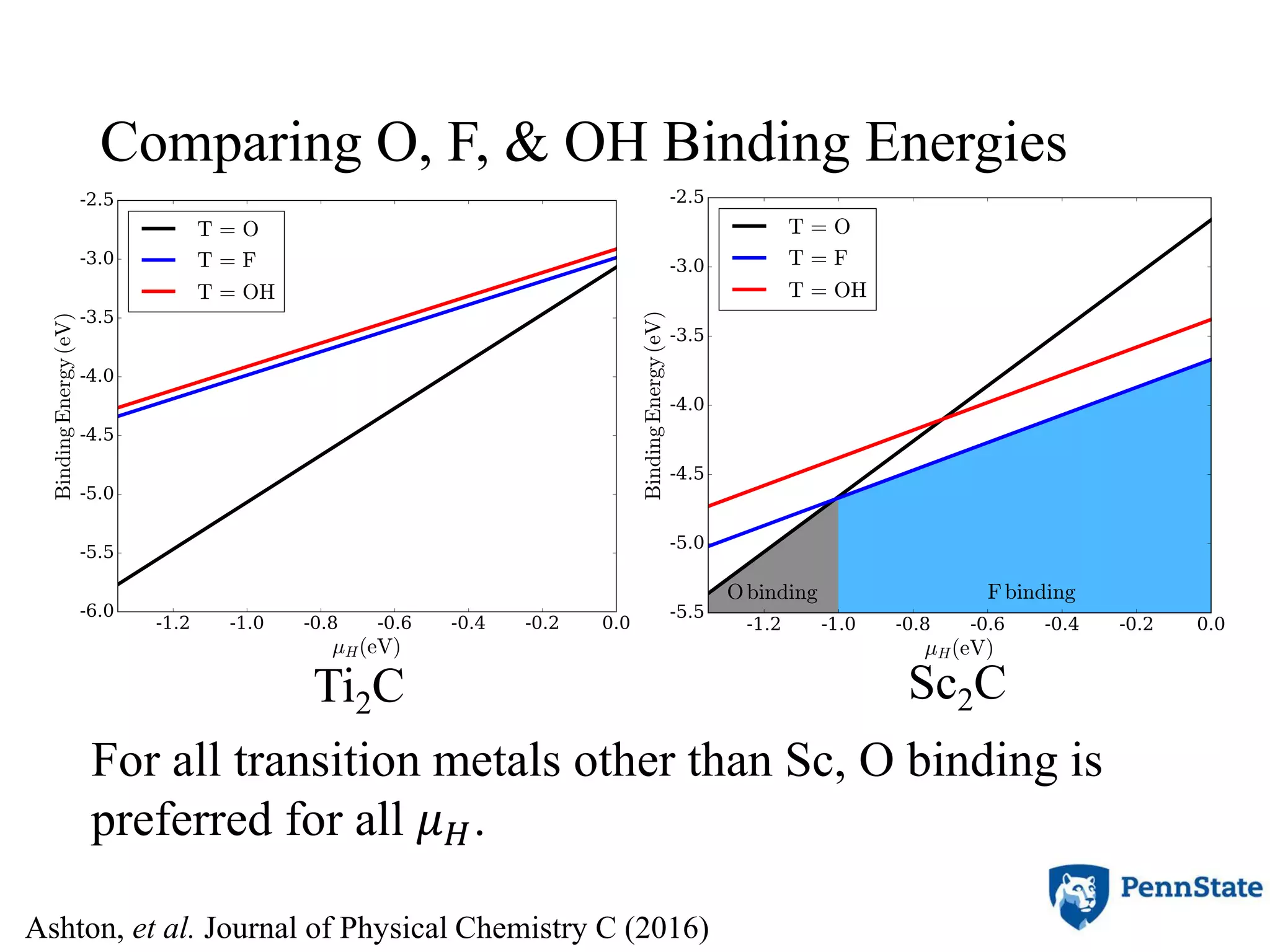
The document discusses the role of atomic-scale modeling in materials design and discovery, highlighting the use of high fidelity computational methods such as quantum chemical approaches and density functional theory. It explores various applications, including the design of M2AX phases, MXenes for battery anodes, and alternatives to rare earth metals in superalloys, emphasizing the importance of understanding fundamental atomic behaviors to inform broader scale models. Additionally, it addresses challenges in predictive modeling, the integration of computational approaches with experimental data, and the need for efficient cyberinfrastructure to facilitate materials research.
![Diffusion Pathways in Multilayer MXenes
10/4/2016 13
O (over)O (under) Li
(a) (b)
[0100]
[1000]
a
b
[1200]
[1100]
Monolayer Multilayer
ΔE (eV)
1.0
2.0
3.0
4.0](https://image.slidesharecdn.com/2016sinnottbrazilmrs-161005210537/75/Role-of-Atomic-Scale-Modeling-in-Materials-Design-Discovery-13-2048.jpg)
![BRI: Searching for RE Alternative
through Crystal Engineering
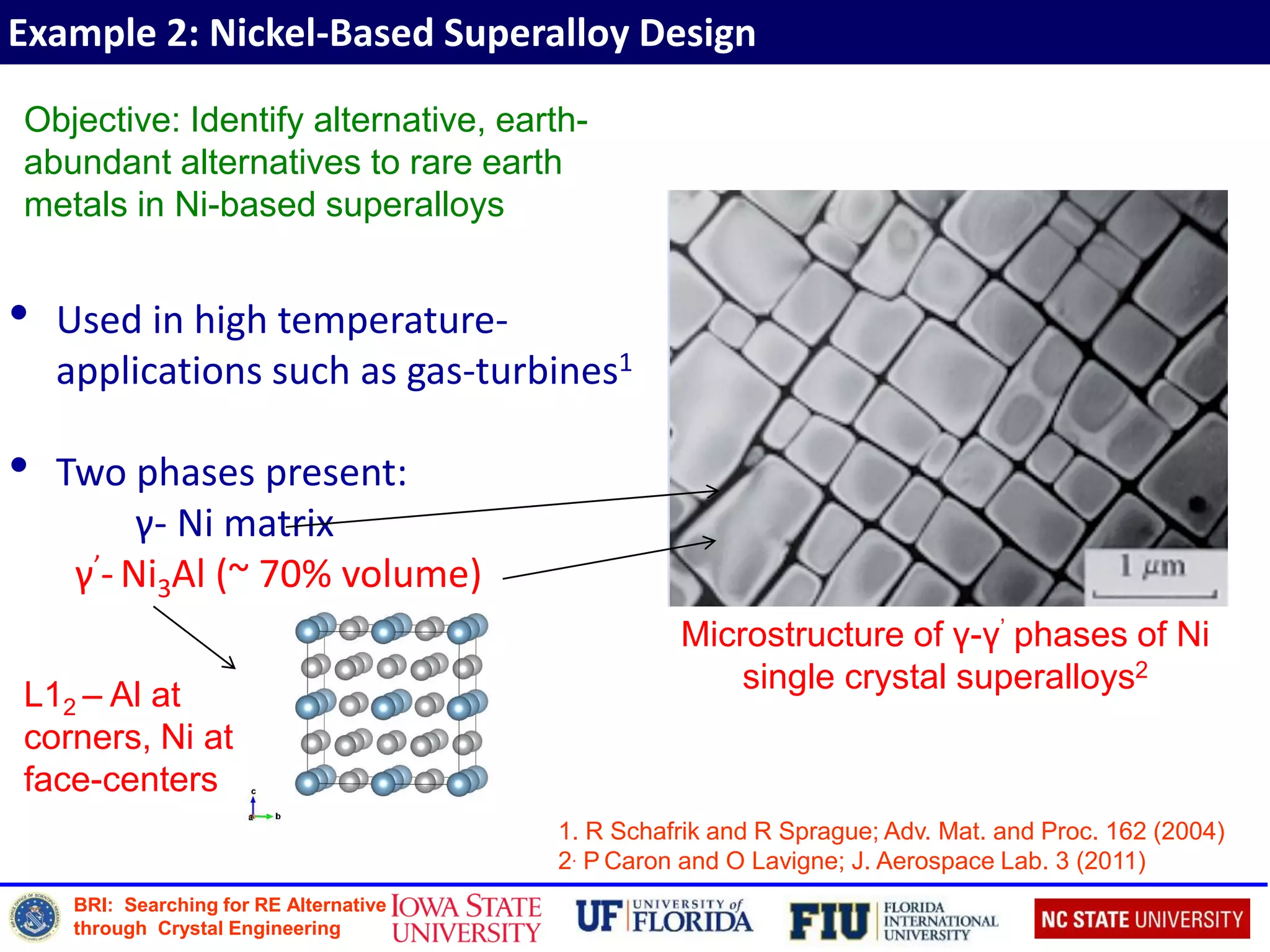
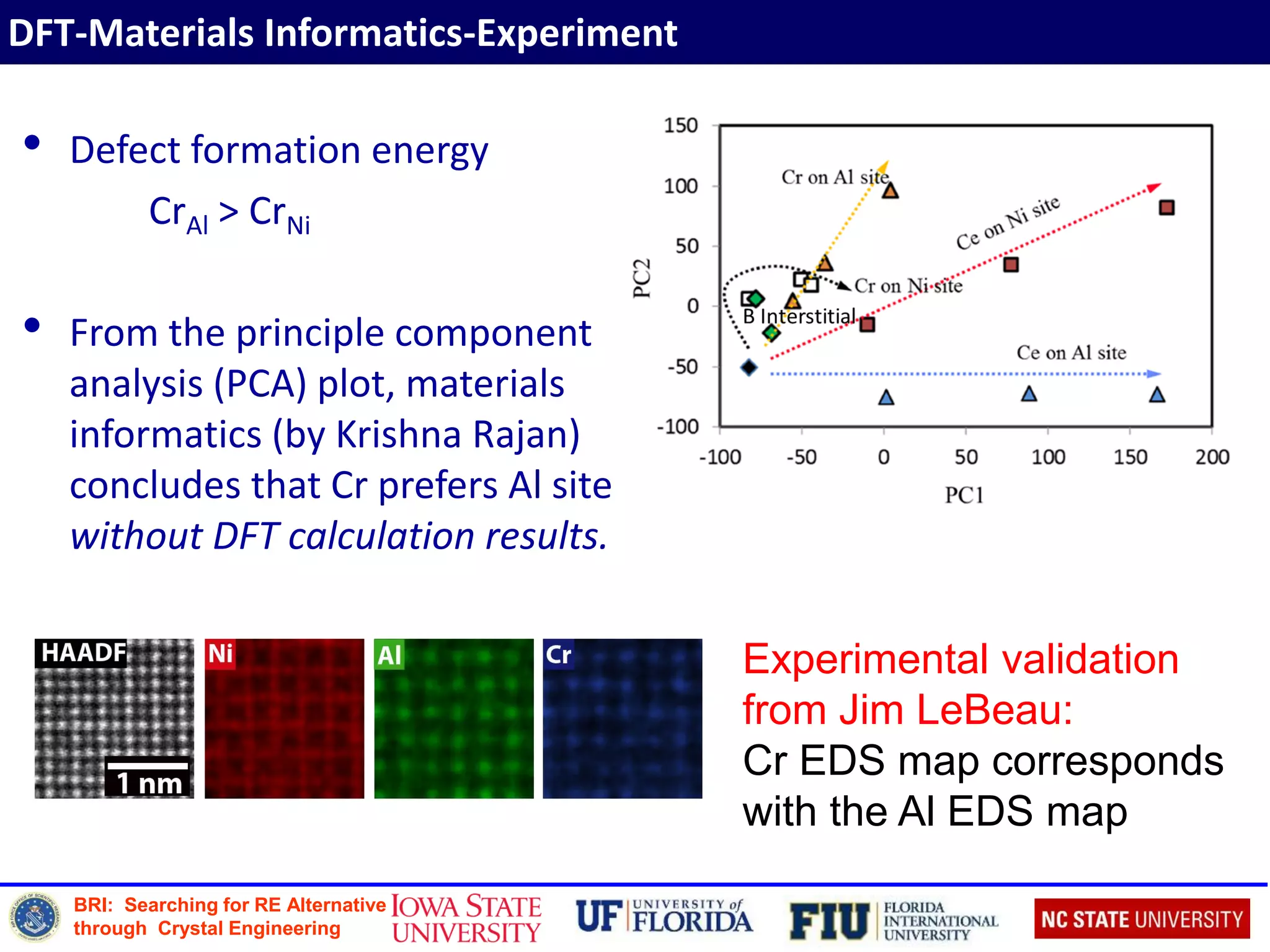
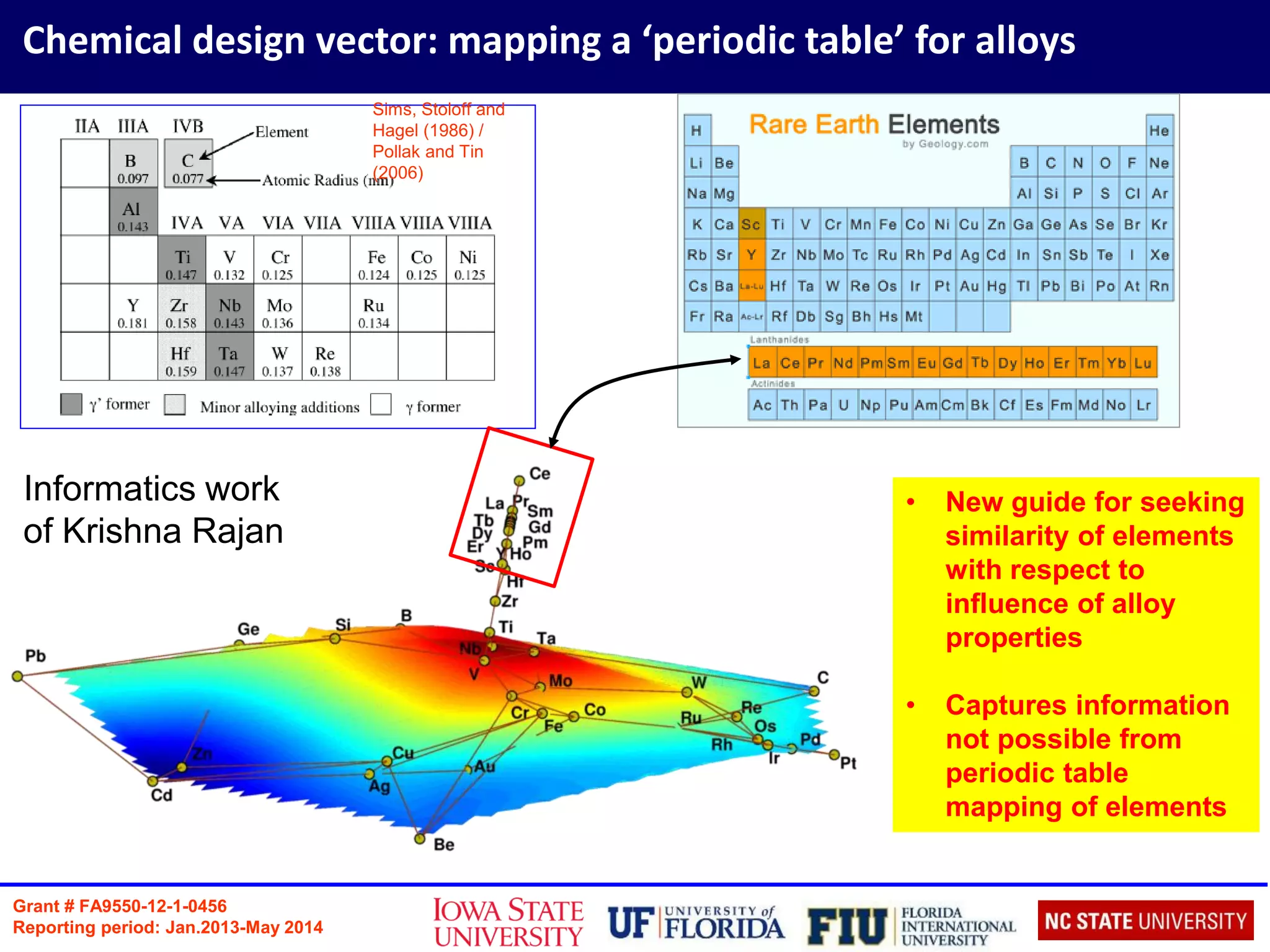
Defect Formation Energy
Defect formation energy of incorporating dopant X is defined as:
Etot[Xq] = total energy of the system with the defect
Etot[bulk] = total energy of the system without the defect
n = number of atoms added (n > 0) or removed (n < 0)
μi = chemical potential of species i
X Ef (XAl) (eV) Ef (XNi) (eV)
B 2.60 0.87
Cr 1.40 (1.351) 0.93 (0.921)
Ce 0.81 1.81
Zr 0.10 (0.041) 0.31 (0.201)
1. D E Kim, S L Shang, Z K Liu; Intermetallics 18 (2010)](https://image.slidesharecdn.com/2016sinnottbrazilmrs-161005210537/75/Role-of-Atomic-Scale-Modeling-in-Materials-Design-Discovery-17-2048.jpg)

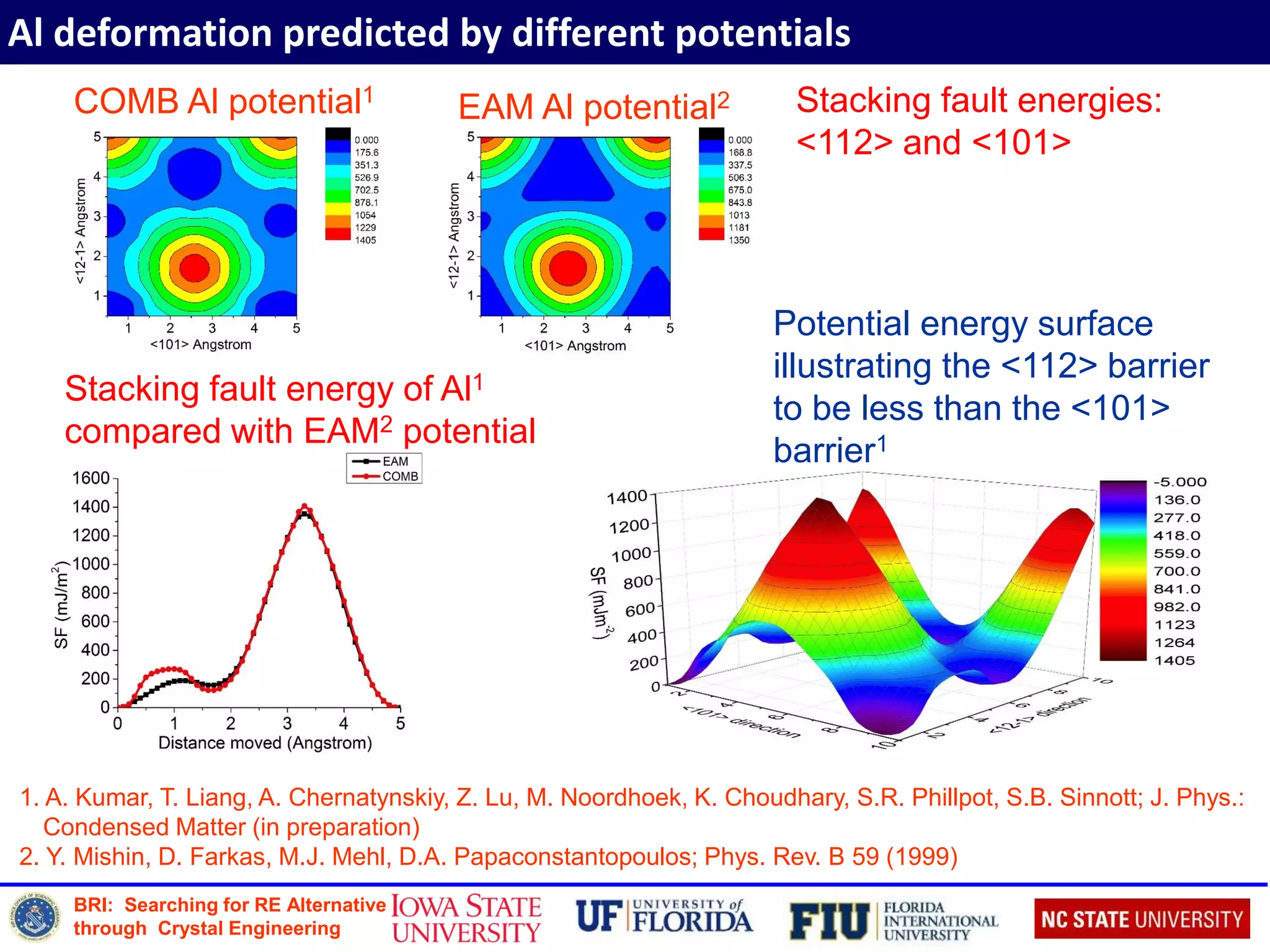
![BRI: Searching for RE Alternative
through Crystal Engineering
Mechanical deformation of Ni3Al at the g/g’ interface
1. A. Kumar, T. Liang, A. Chernatynskiy, Z. Lu, M. Noordhoek, K. Choudhary, S. R. Phillpot, S.B. Sinnott (in
preparation)
2. M.H. Yoo, M.S. Daw, M.I. Baskes, V. Vitek, D.J. Srolovitz, Eds.; New York: Plenum Press; 1989. p. 401.
Thermostat
Active
Rigid moving
Rigid moving
Thermostat
Active
Ni3Al
Ni
τzx
Z
[010]
X
[101]
Y
[10 -1]
τzx
• Edge dislocations at the Ni-Ni3Al interface
• Predict mechanisms associated with
applied shear stress and dislocation
motion
Ni3Al Ec
(eV/atom)
B
(GPa)
G
(GPa)
COMB1 -4.61 198 93
exp.2 -4.62 195 96
Simulation box size:
16.67x16.67x9.21 nm3
Total number of
atoms: 179,600
Dislocation](https://image.slidesharecdn.com/2016sinnottbrazilmrs-161005210537/75/Role-of-Atomic-Scale-Modeling-in-Materials-Design-Discovery-27-2048.jpg)