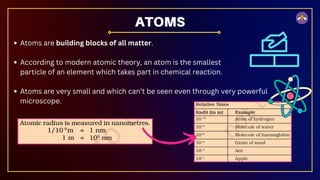
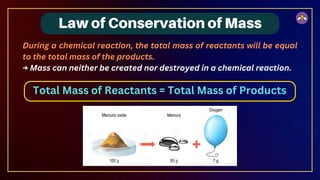
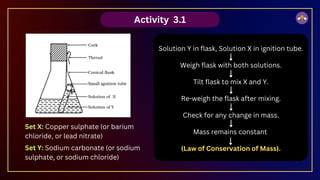
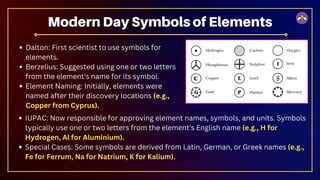
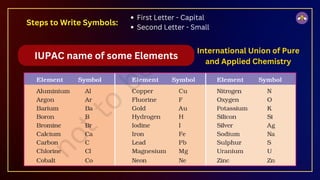
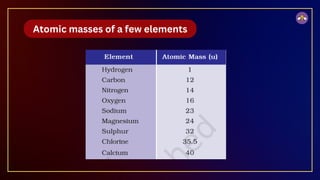
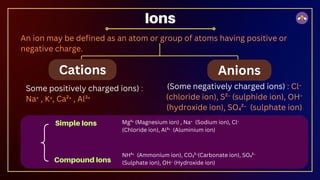
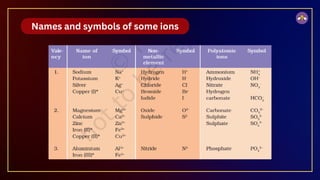
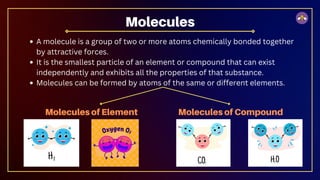
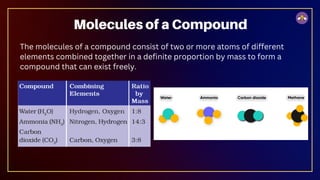
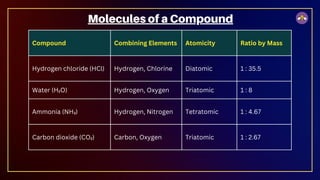
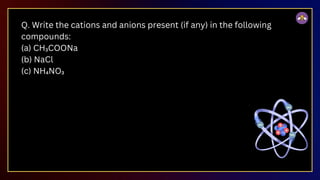
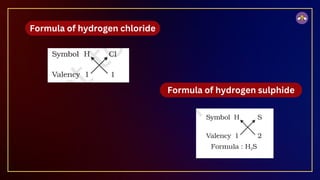
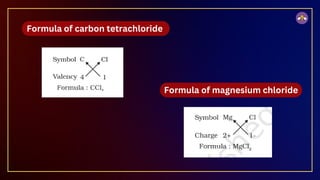
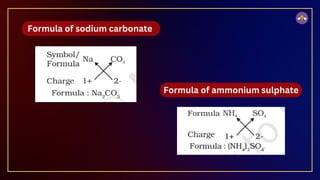
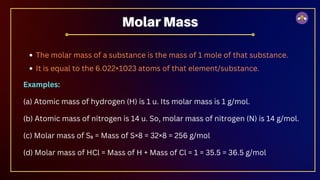
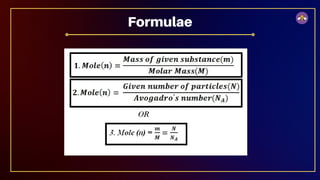
The document outlines fundamental concepts in chemistry, covering atomic theory, laws of chemical combination, atomic mass, and molecular formulas. It discusses the principles established by early scientists like Dalton, Lavoisier, and Proust, emphasizing the conservation of mass and the law of constant proportions. Additionally, it explains the modern interpretation of elements, atoms, ions, and their interactions to form compounds, as well as the mole concept and how to calculate molar masses.