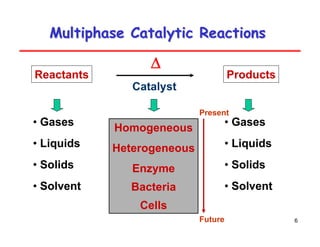
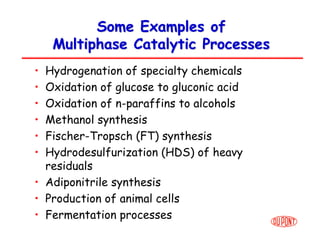
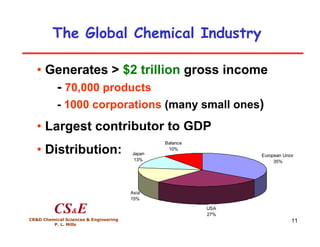
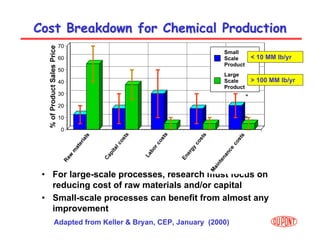
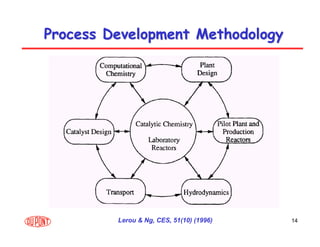
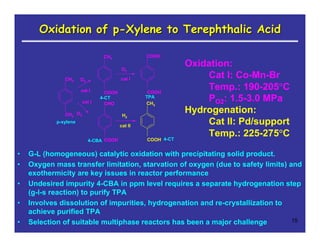
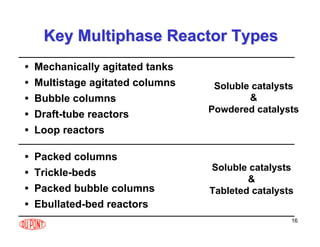
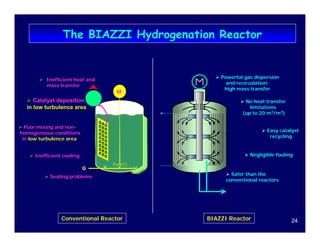
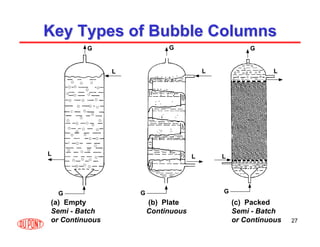
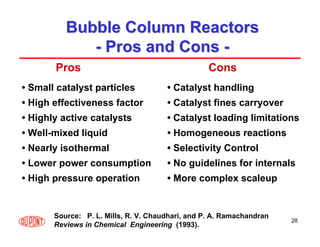
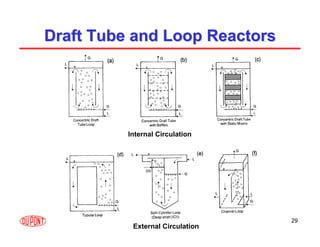
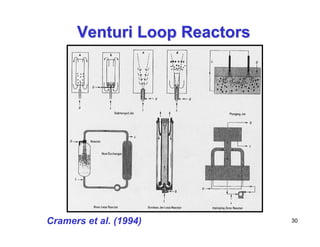
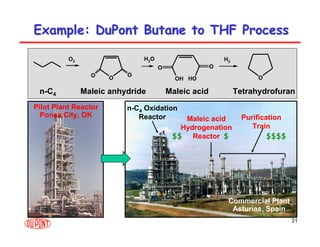
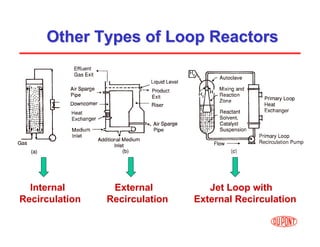
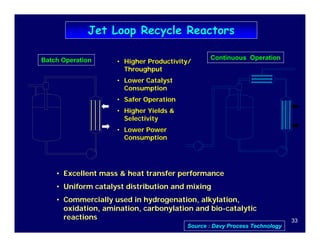
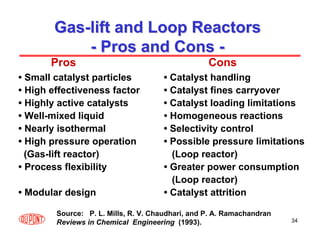
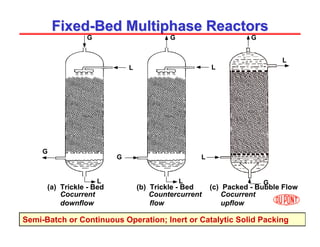
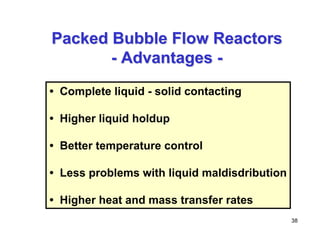
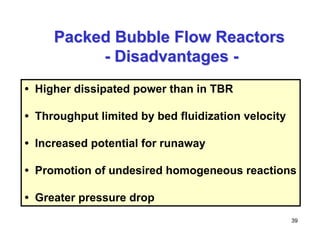
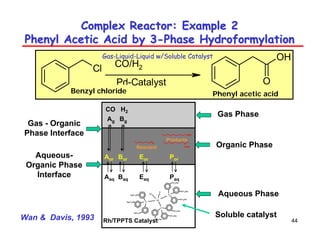
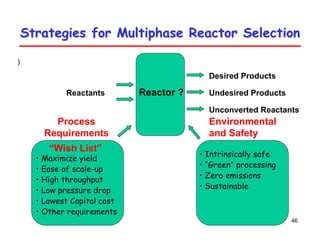
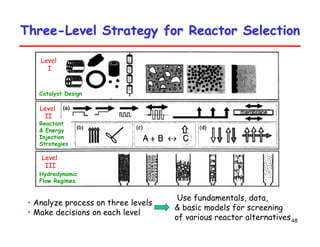
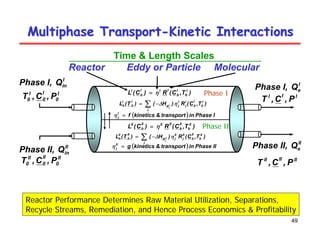
This document provides an overview of heterogeneous reactors and application areas. It outlines the course objectives which are to review basic multiphase reaction engineering, develop relationships between transport effects and kinetics, derive performance equations for various reactor types, and present case studies. The document then discusses various reactor types including mechanically agitated tanks, bubble columns, trickle beds, and loop reactors. It provides examples of industrial multiphase catalytic processes and emerging technologies.