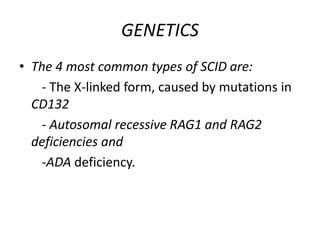
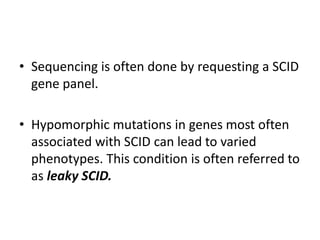
This document discusses severe combined immunodeficiency (SCID), a group of genetic disorders caused by mutations that lead to absence of T- and B-cell function. Patients with SCID have very small or absent lymph nodes and spleen and are susceptible to life-threatening infections from a variety of pathogens. While newborn screening has improved early detection, SCID often presents with infections in the first year of life without hematopoietic stem cell transplantation or gene therapy. The document outlines pathogenesis, clinical manifestations, diagnosis, treatment options including stem cell or gene therapy, and genetics of the most common forms of SCID.