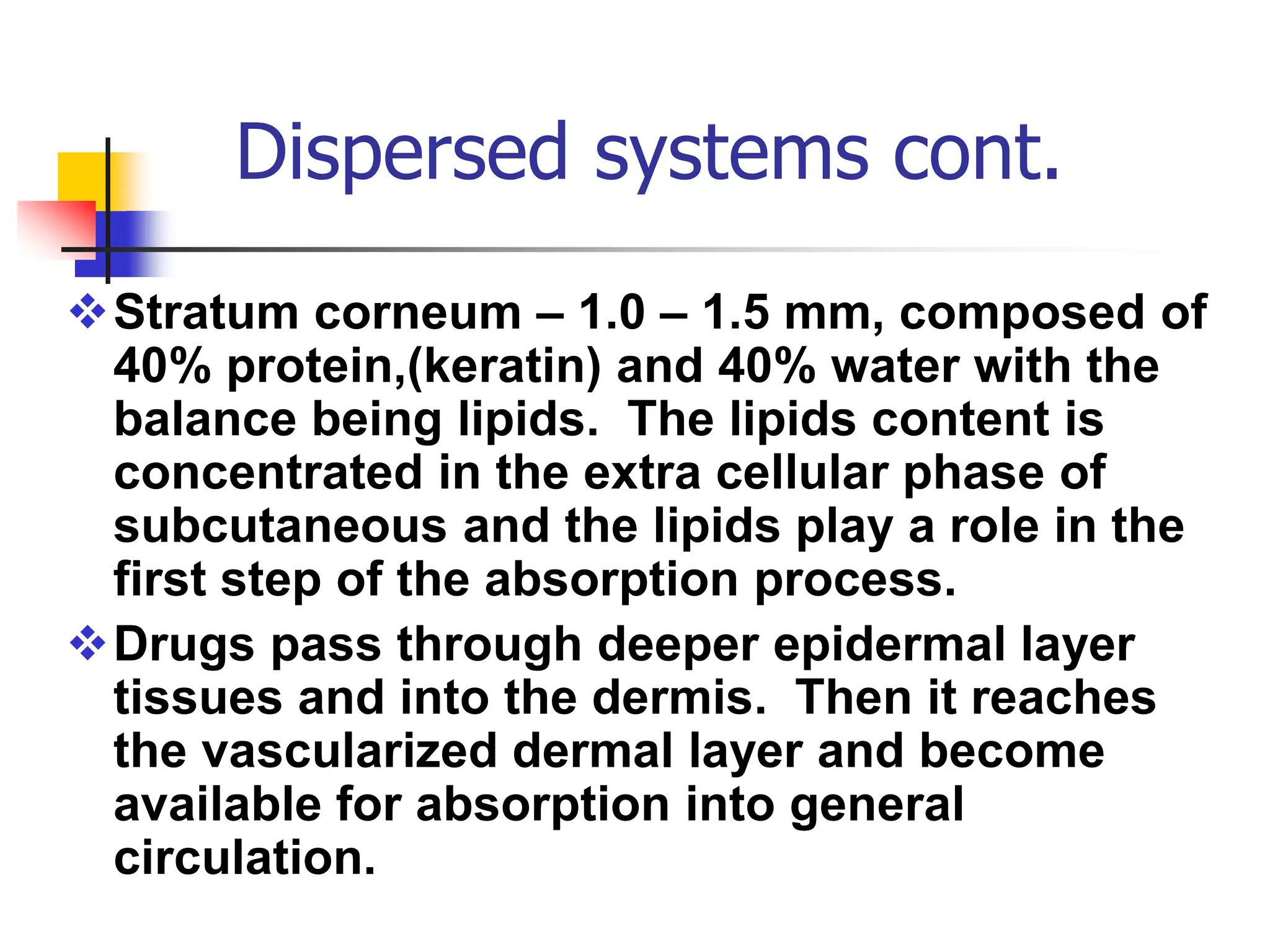
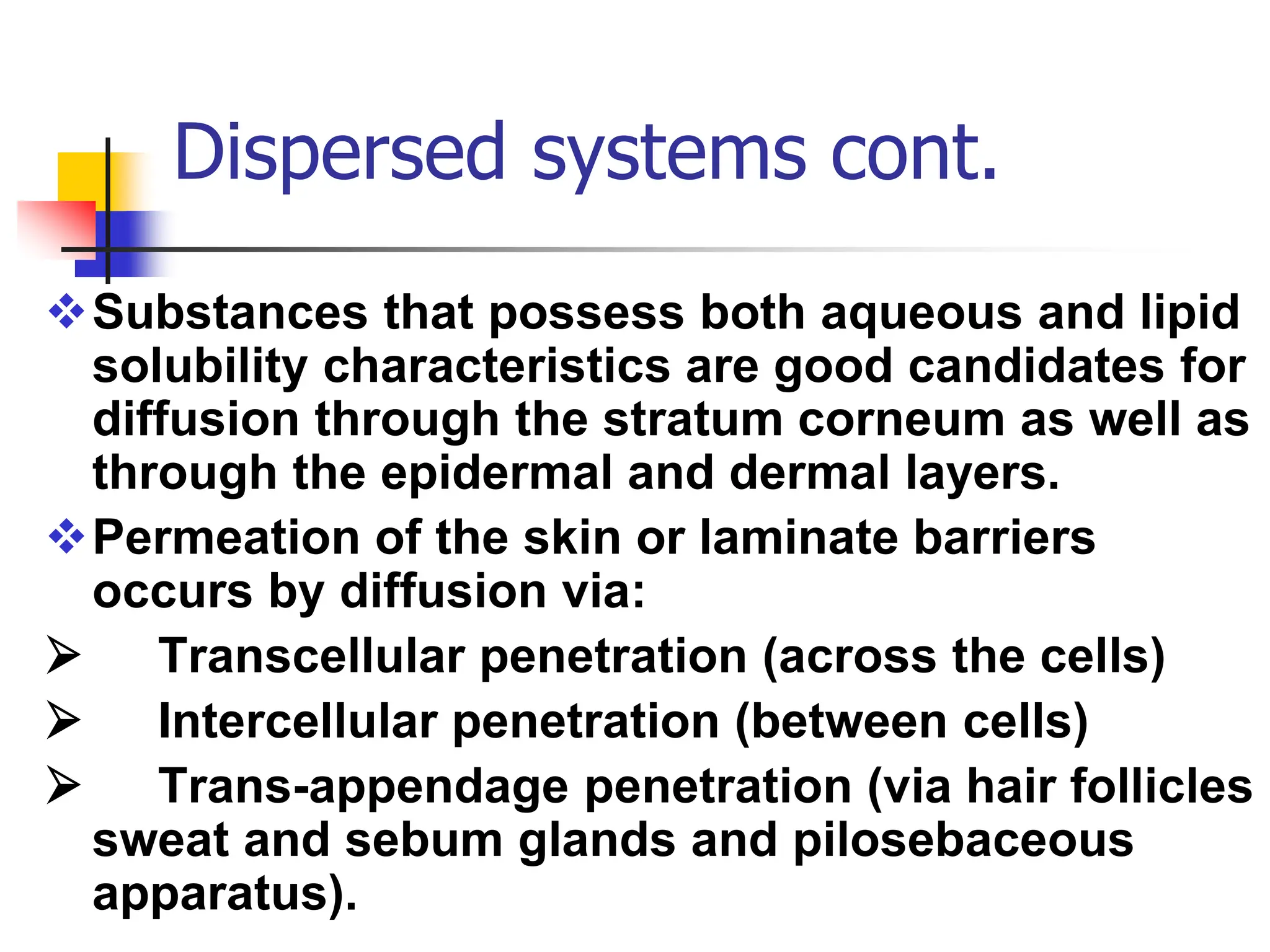
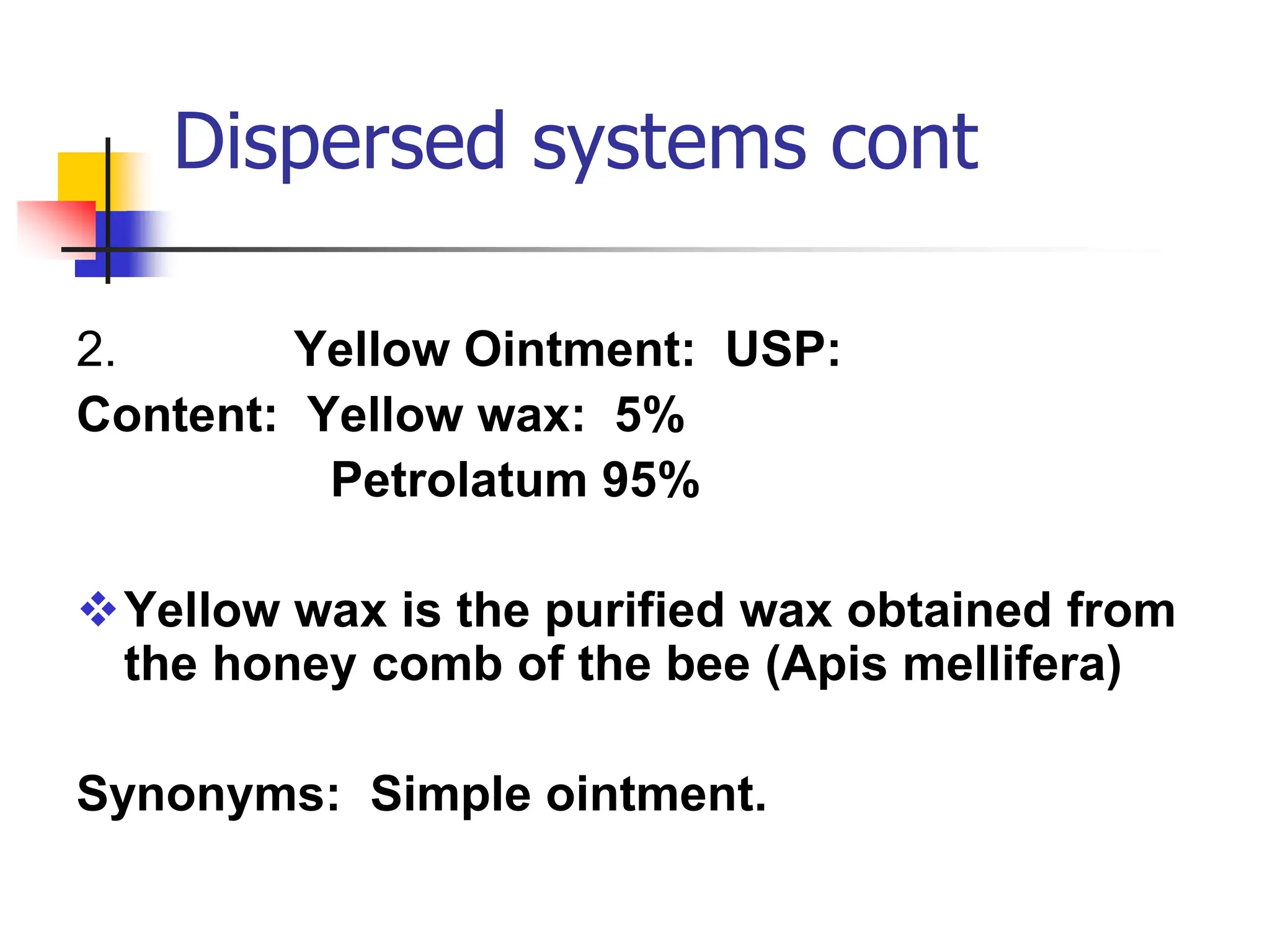
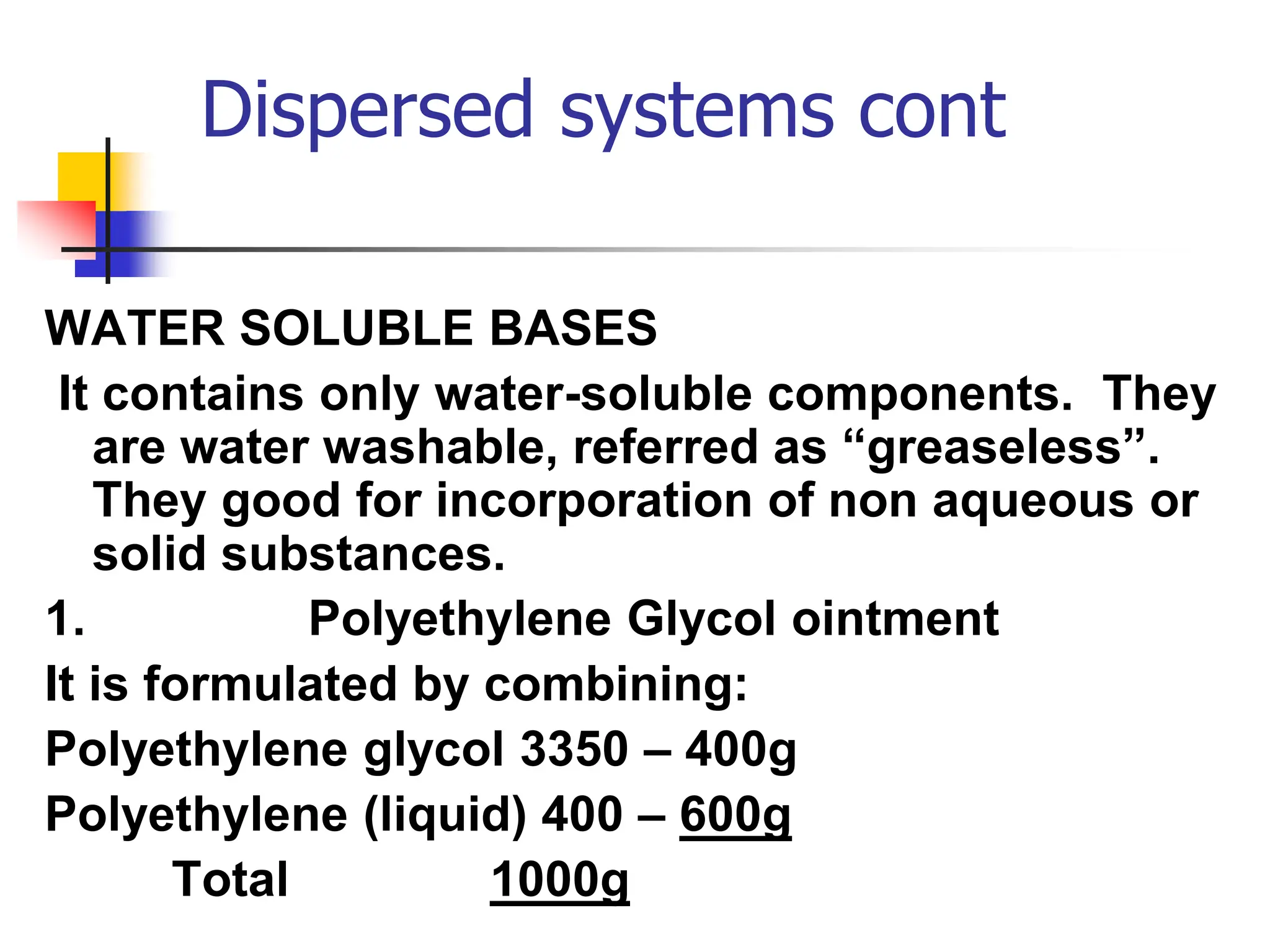
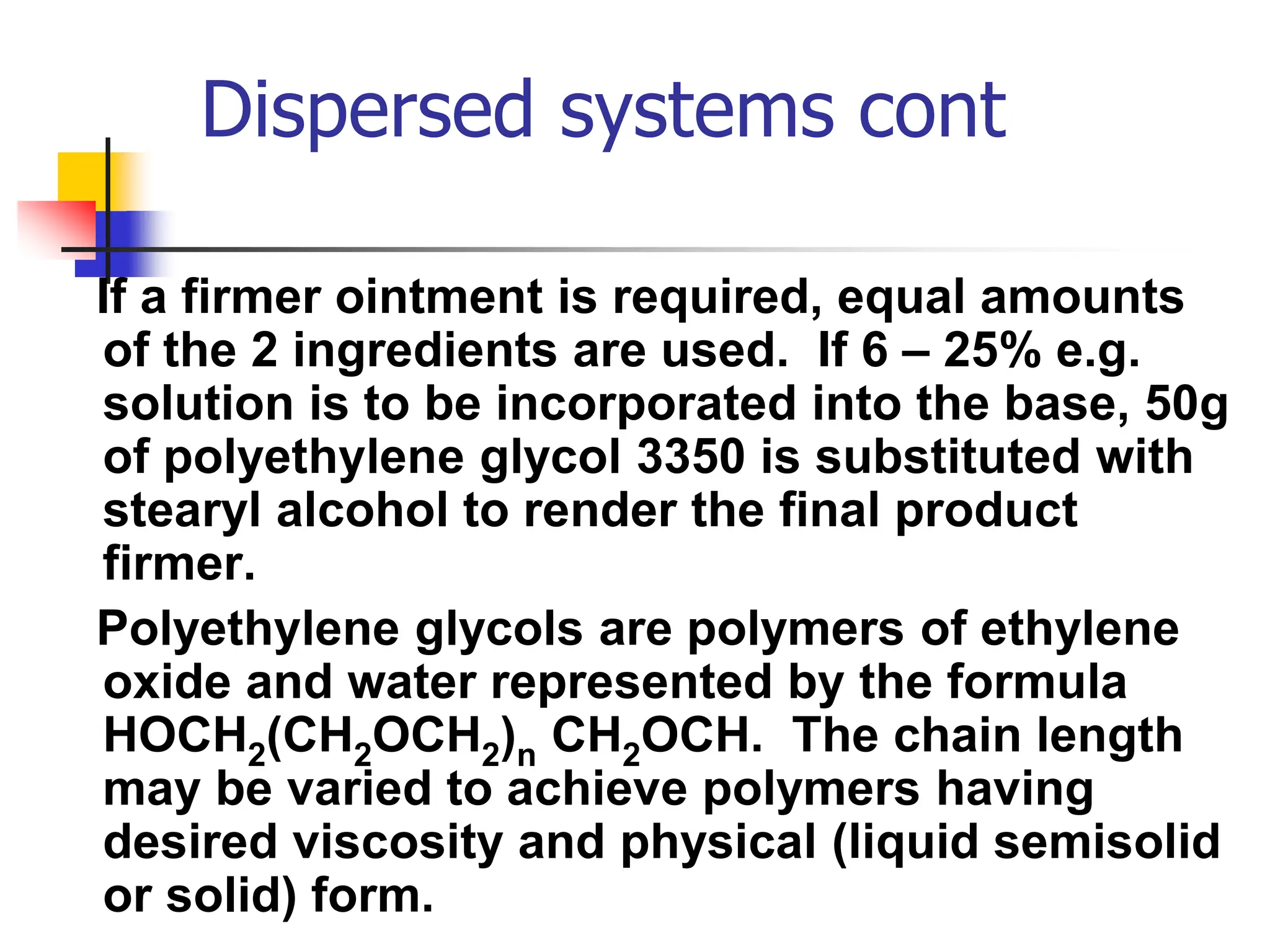
The document discusses transdermal drug delivery systems, primarily focusing on semi-solid dispersed systems such as ointments, creams, and lotions used for topical application. It elaborates on the properties, formulation methods, and factors affecting percutaneous absorption of drugs through the skin, addressing the significance of drug-vehicle combinations and skin conditions. Additionally, it categorizes ointment bases into hydrocarbon, absorption, water-removable, and water-soluble bases, detailing their characteristics and applications.