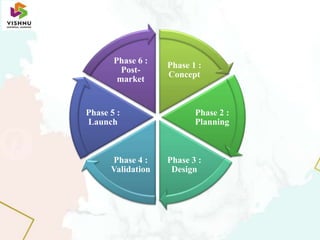
The document discusses the product lifecycle of medical devices. It begins with an introduction that defines the basic phases of a product lifecycle as research, development, production, and end of life. It then outlines 6 key phases in the medical device product lifecycle: 1) Concept, 2) Planning, 3) Design, 4) Validation, 5) Launch, and 6) Post-market. Each phase is described in 1-2 sentences. For example, the Concept phase involves initially defining the product and exploring funding/routes to market, while the Post-market phase includes ongoing surveillance and reporting to continuously evaluate safety.