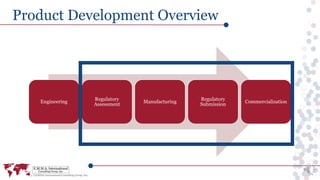
This document provides an overview of a webinar discussing efficient medical device product development processes that align quality, regulatory, and business needs. It emphasizes the importance of defining product requirements, understanding regulatory submissions, and selecting compliant manufacturers. Additionally, it covers post-commercialization considerations and how experts can assist in navigating the complexities of medical device development.






![Product Requirements:
• Will define device’s
intended use, features,
and functionality
• Requirements should be
specific and measurable
• Will tie into design
controls and User Needs
traceability[1]
Regulatory Assessment: Developing Requirements
This Photo by Unknown Author is licensed under CC BY-SA-NC](https://image.slidesharecdn.com/productdevelopmentwebinarv2-200411194600/85/Medical-Device-Product-Development-8-320.jpg)

![Regulatory Assessment: Planning for FDA Submission
• Determine what premarket
submission type, if any, is required
for your device
• 510(k) (Premarket Notification)
• PMA (Premarket Approval)
• De Novo Classification Request
• Determine what requirements your
device will have to comply with[2]
• Design Controls
• Clinical Testing
This Photo by Unknown Author is licensed under CC BY-SA-NC](https://image.slidesharecdn.com/productdevelopmentwebinarv2-200411194600/85/Medical-Device-Product-Development-10-320.jpg)

![Manufacturing:Various Requirements
• There are several different types
of assembly and environments
your device may require
• ISO 14644-1 Cleanrooms and
associated controlled
environments[3]
• Overmolding for medical devices
• Medical device assembly
precision
• A robust QMS will need to be
built for device manufacturing](https://image.slidesharecdn.com/productdevelopmentwebinarv2-200411194600/85/Medical-Device-Product-Development-12-320.jpg)


![Regulatory Submission: Acceptance Review
• “Is the Submission with the
appropriate Center?”
• “Submission contains a Table of
Contents”
• “Each Section is Labeled”
• File type is also important (PDF vs.
Word)
• Each file must follow a specific
naming convention
• 180 day deadline for re-
submission[4]
This Photo by Unknown Author is licensed under CC BY-SA](https://image.slidesharecdn.com/productdevelopmentwebinarv2-200411194600/85/Medical-Device-Product-Development-15-320.jpg)

![Commercialization
• MDSAP: Australia, Brazil, Canada,
Japan, U.S.
• Harmonized to 13485: 2016[5]
• More robust than 21 CFR 820
• More ties to ISO 14971 (Risk
Management)[6]](https://image.slidesharecdn.com/productdevelopmentwebinarv2-200411194600/85/Medical-Device-Product-Development-17-320.jpg)
![[7]](https://image.slidesharecdn.com/productdevelopmentwebinarv2-200411194600/85/Medical-Device-Product-Development-18-320.jpg)


