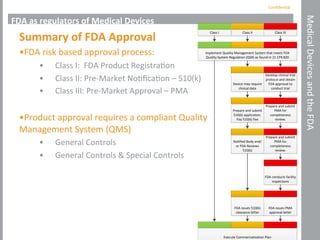
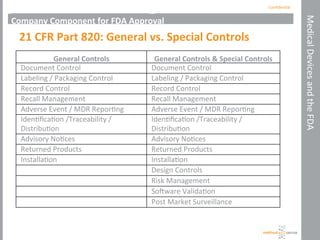
This document outlines the FDA regulations and pathways for medical devices, explaining the definitions, classification, and approval processes involved. It describes the roles of the FDA, specifically the Center for Devices and Radiological Health, in regulating medical devices and includes details on product and company components of FDA approval. The document emphasizes the importance of quality management systems for manufacturers and highlights various approval pathways, such as the 510(k) process and De Novo applications.