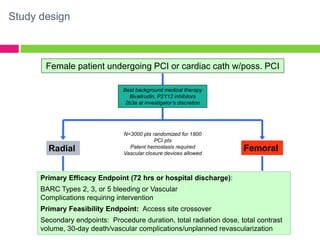
The SAFE-PCI for Women Trial was a prospective, randomized trial comparing radial versus femoral approaches for percutaneous coronary intervention (PCI) in women. 1787 women undergoing cardiac catheterization or PCI were randomized to radial or femoral access. The trial was terminated early due to lower than expected event rates. In the subgroup of women undergoing PCI (n=345 radial, n=346 femoral), there was no significant difference in the primary efficacy endpoint of bleeding or vascular complications between radial and femoral access. However, radial access was associated with a higher rate of needing conversion to femoral access. Overall, the results suggest an initial radial access strategy may be preferred for some women undergoing cardiac procedures.