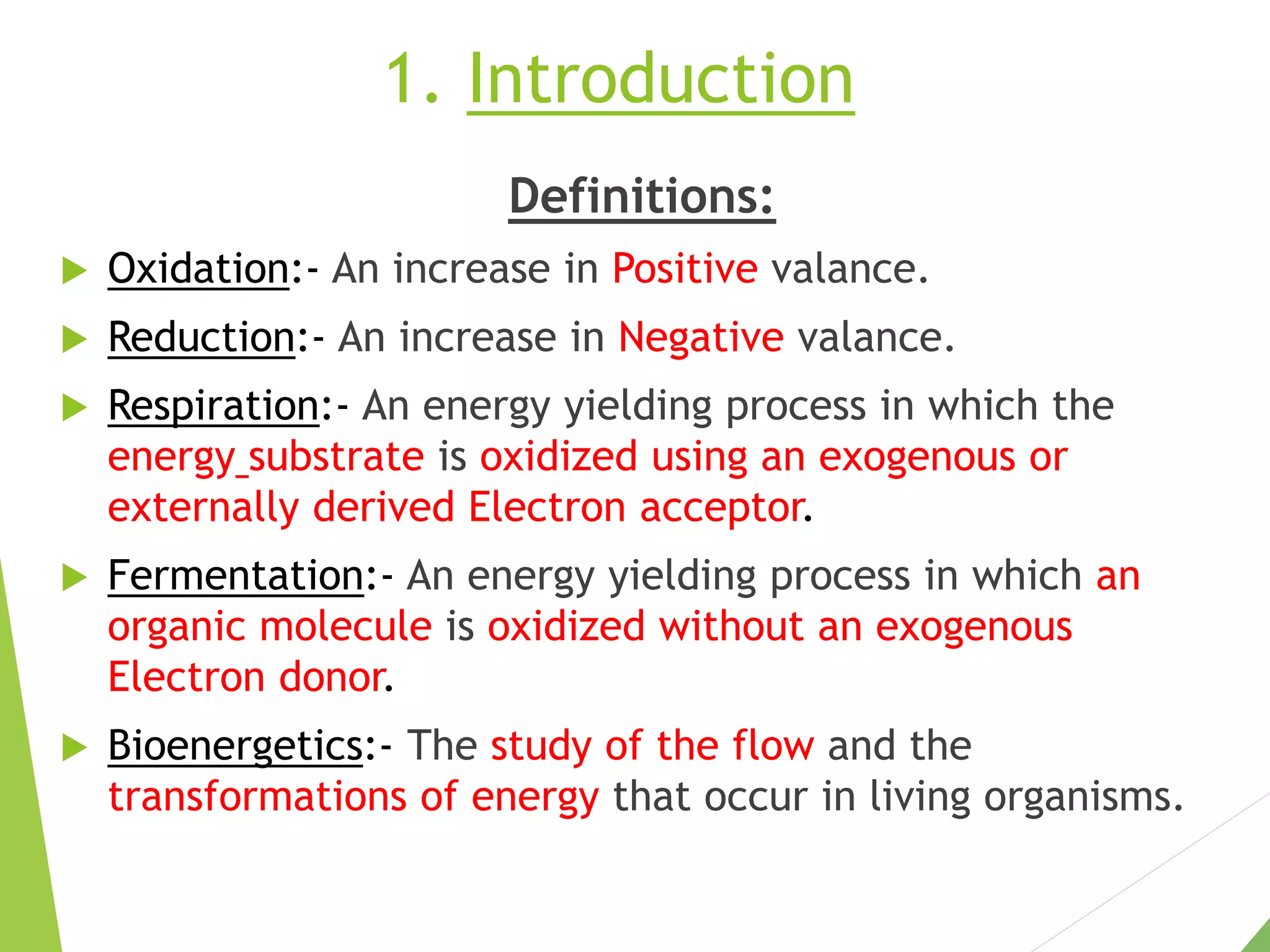
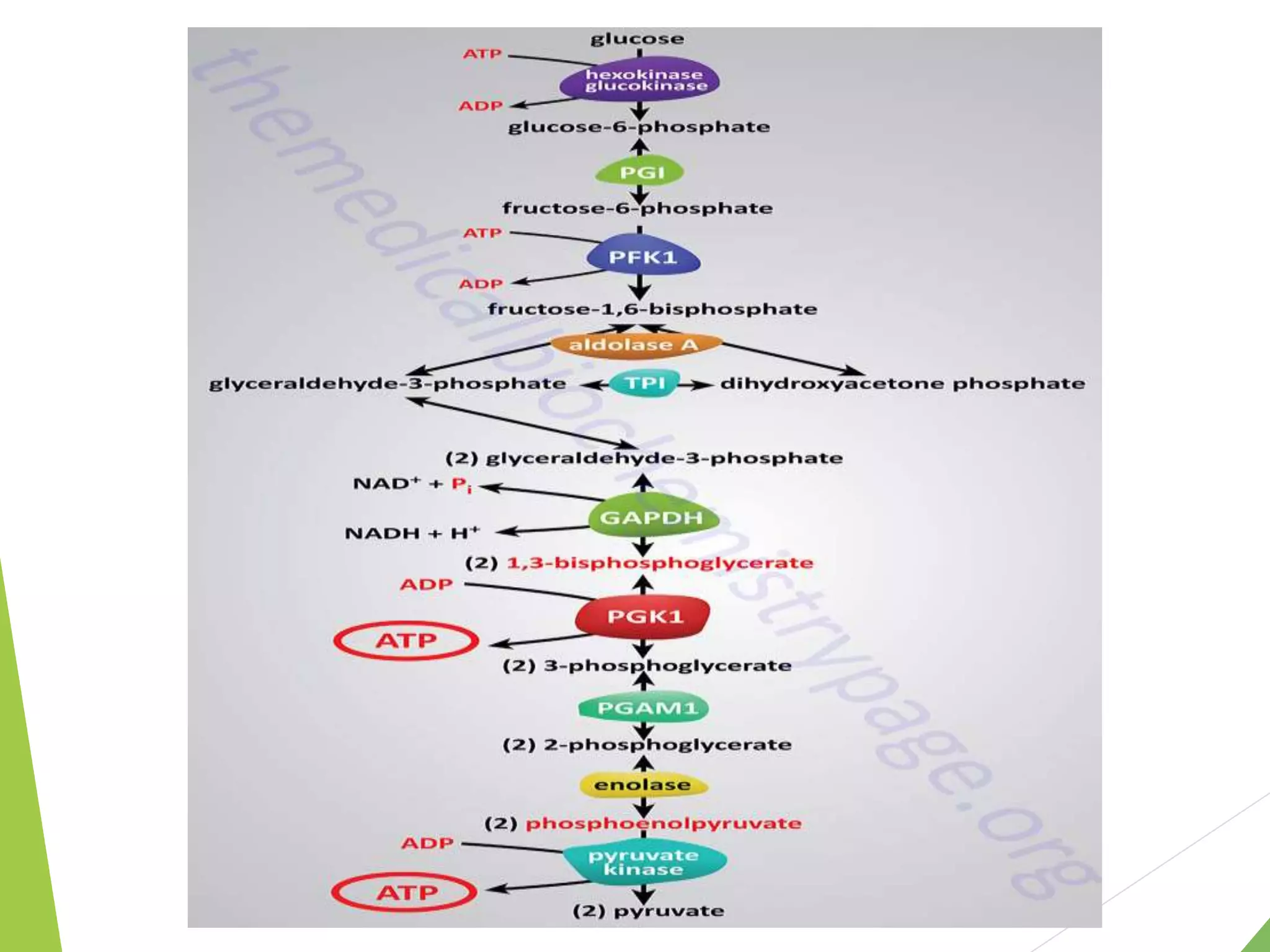
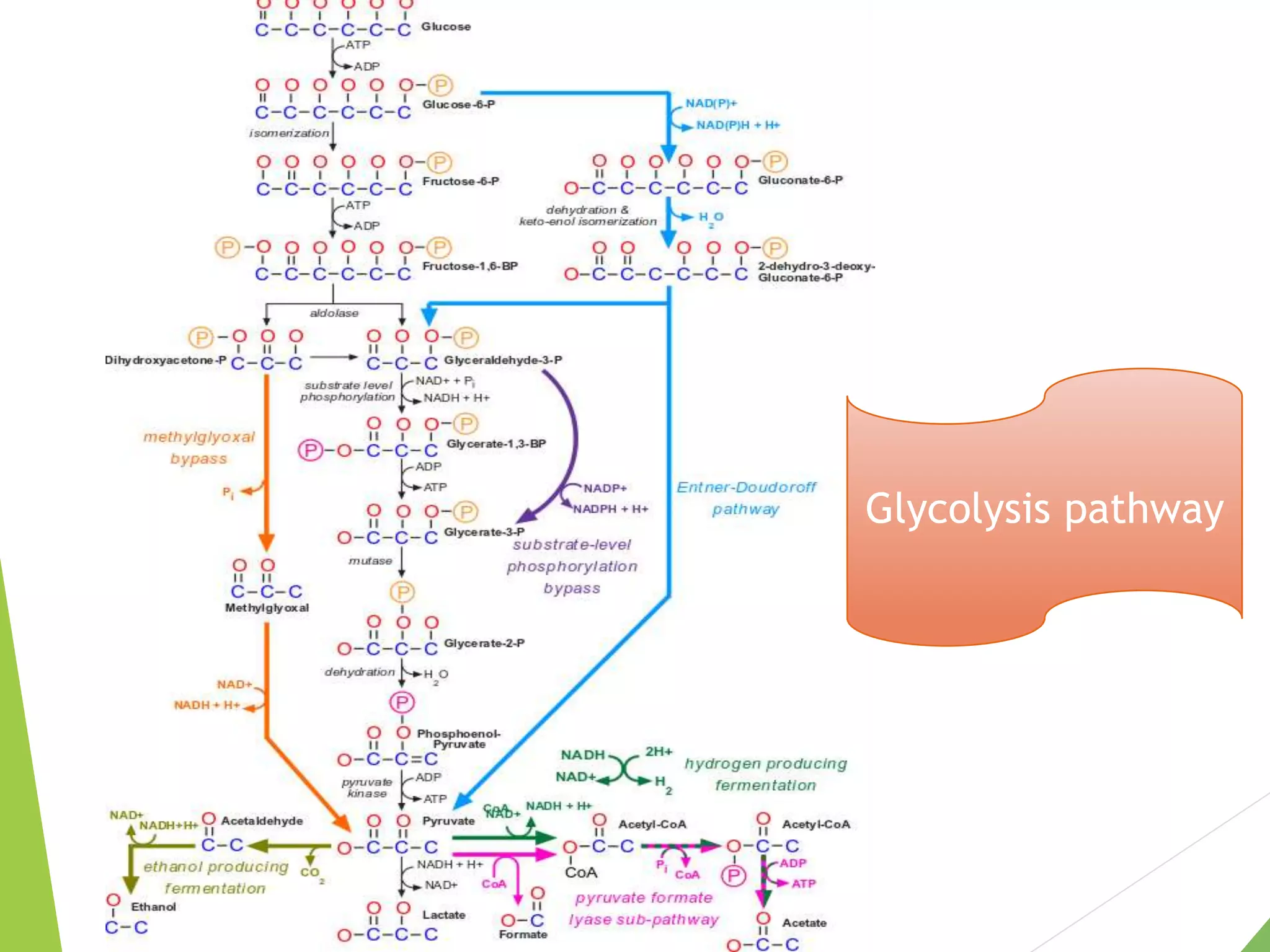
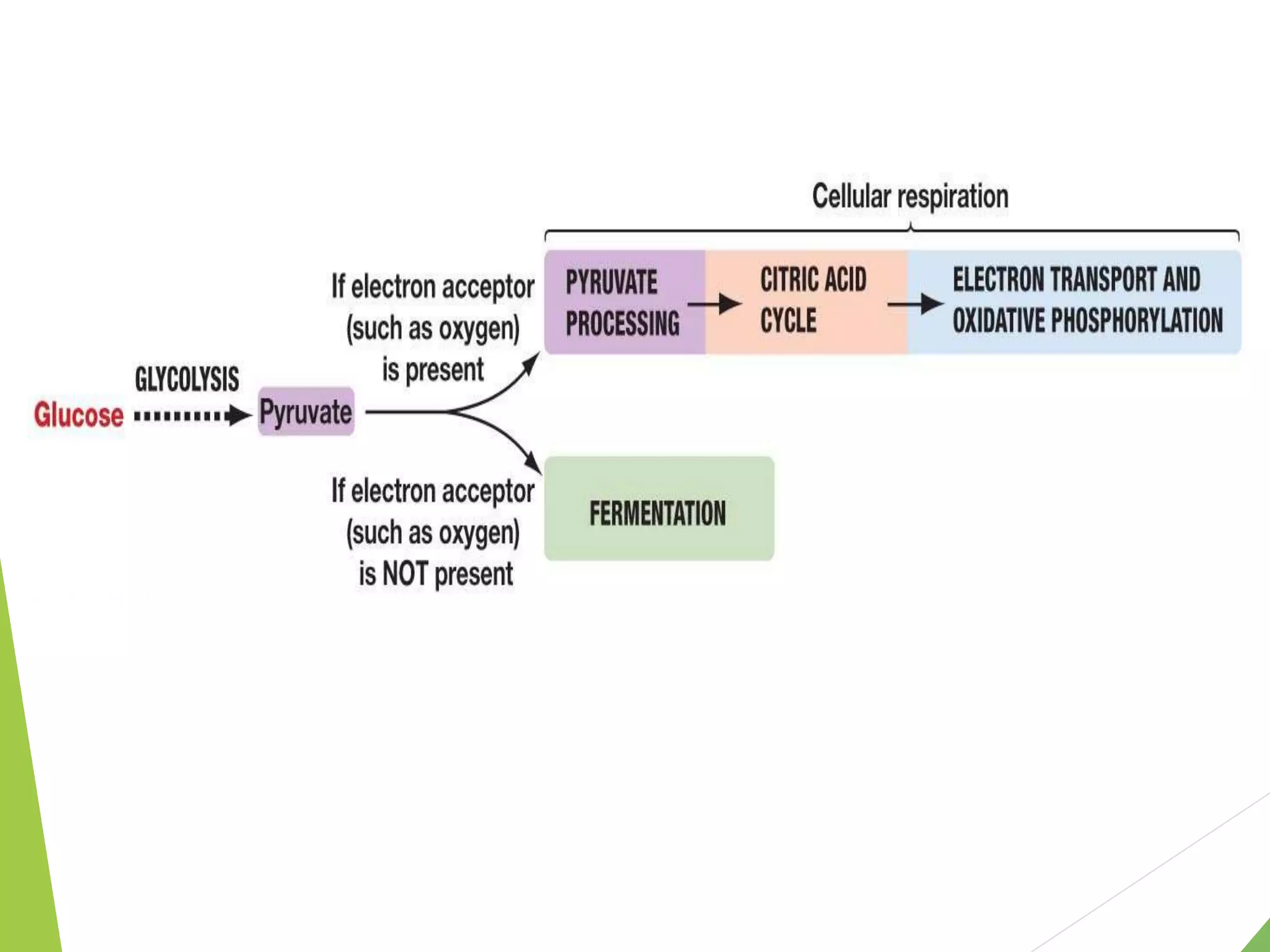
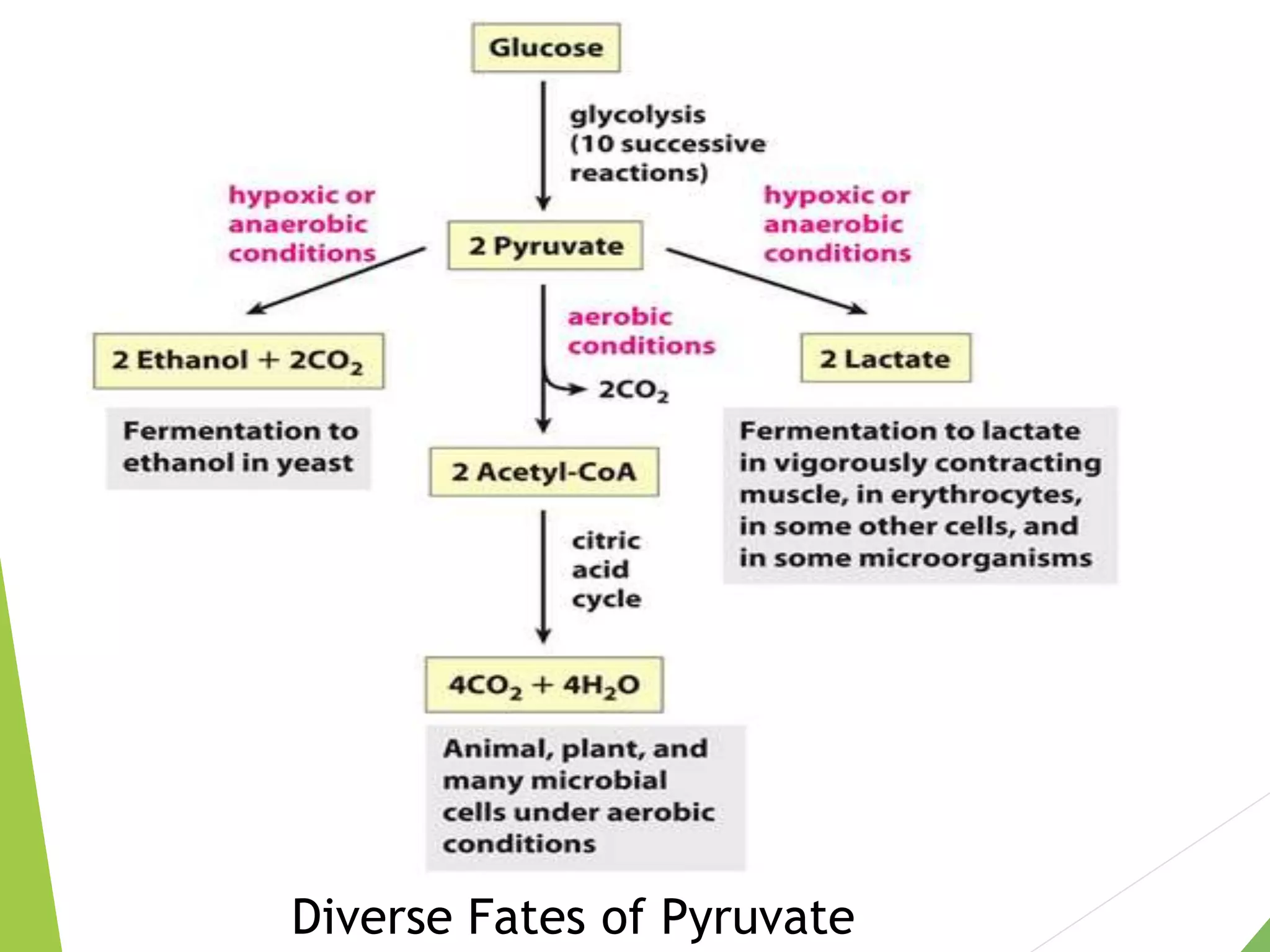
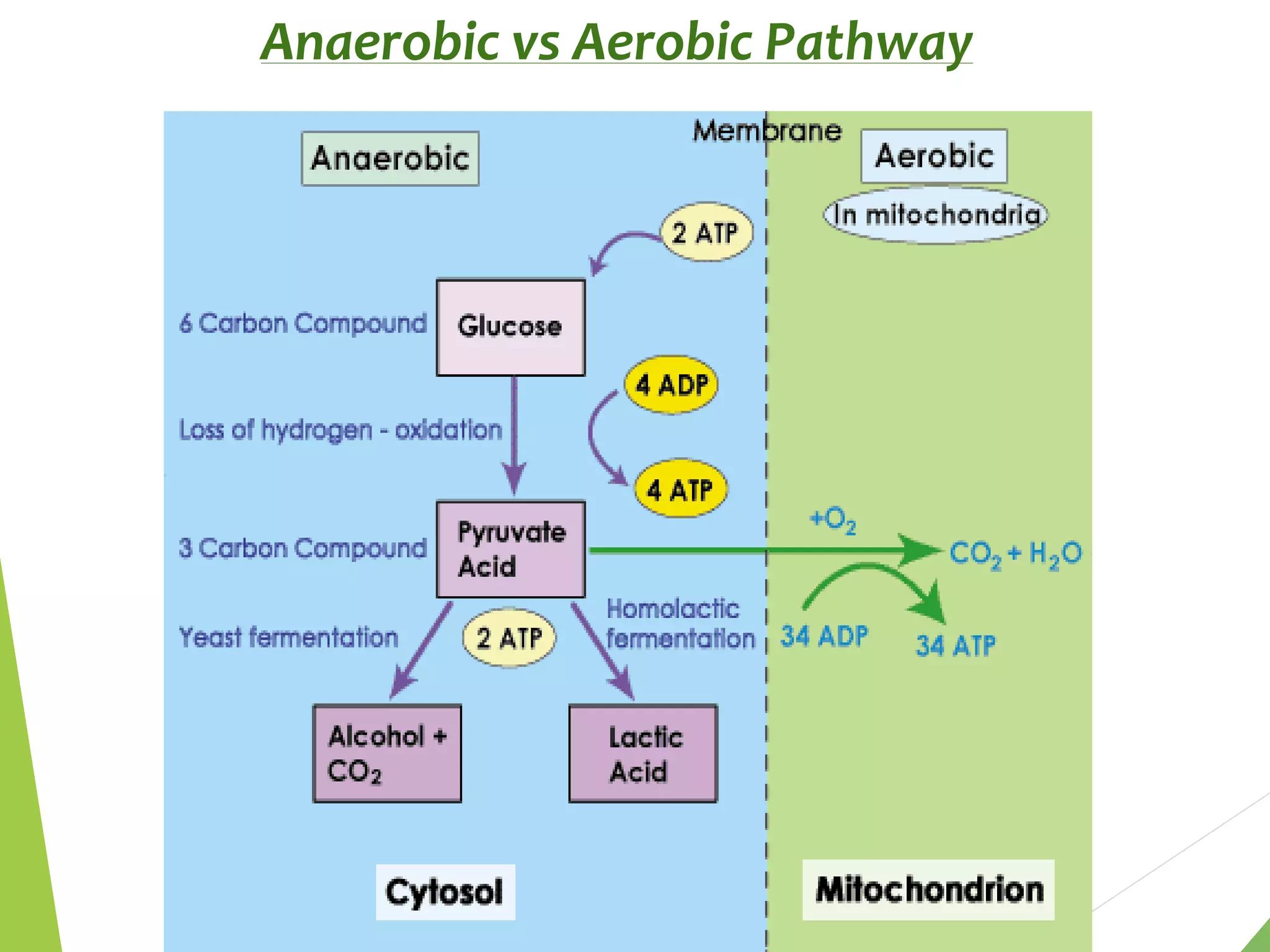
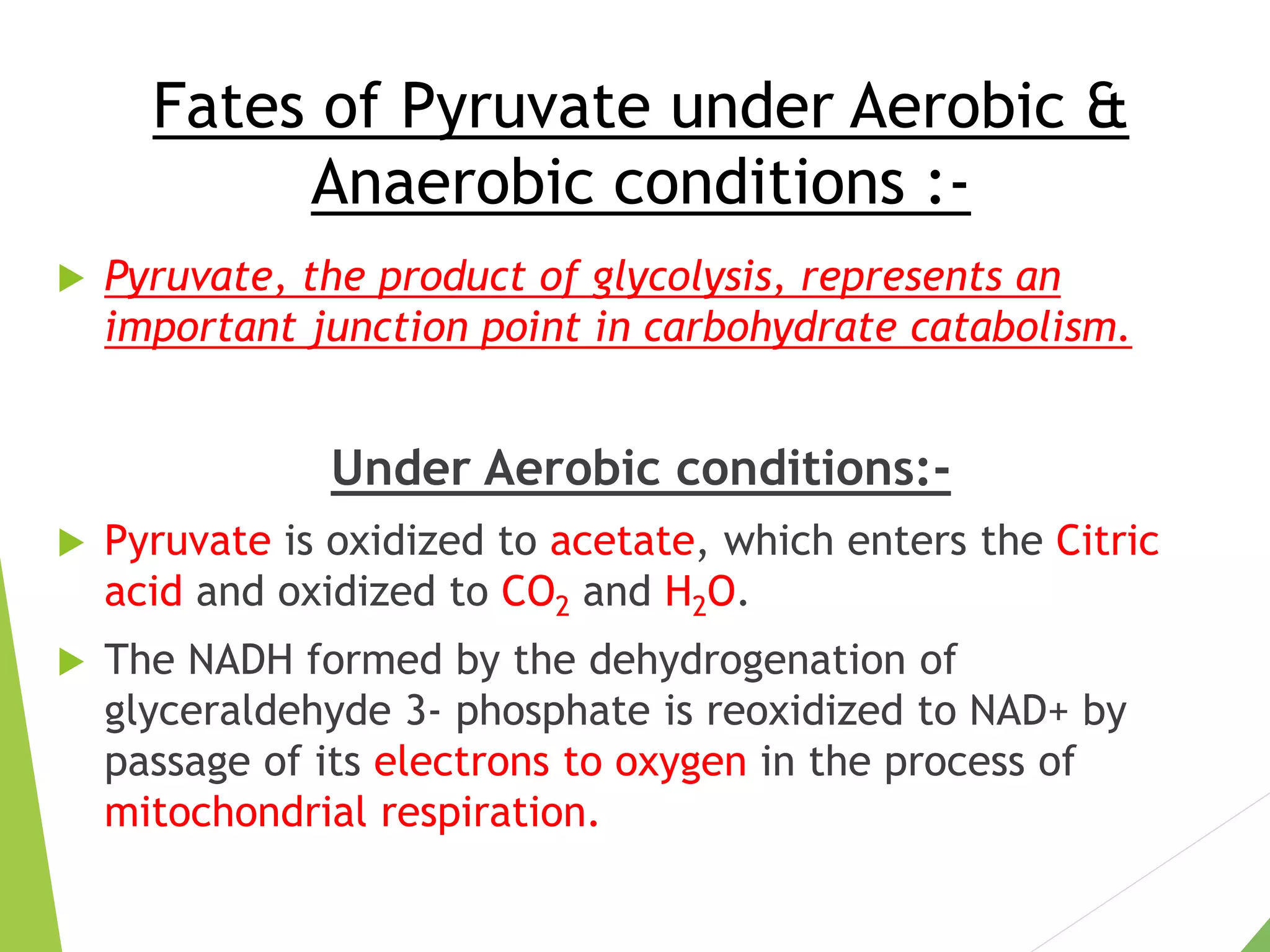
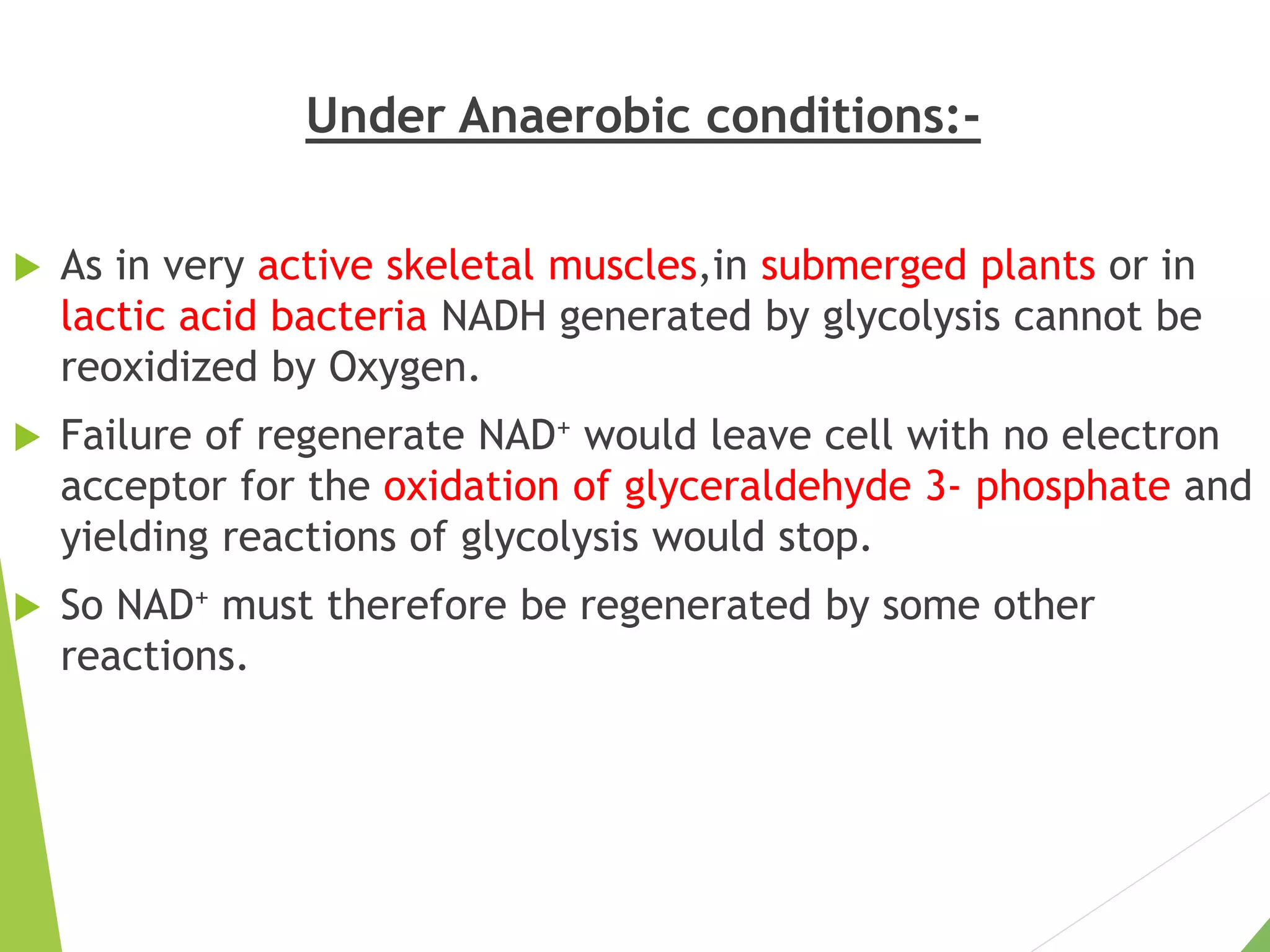
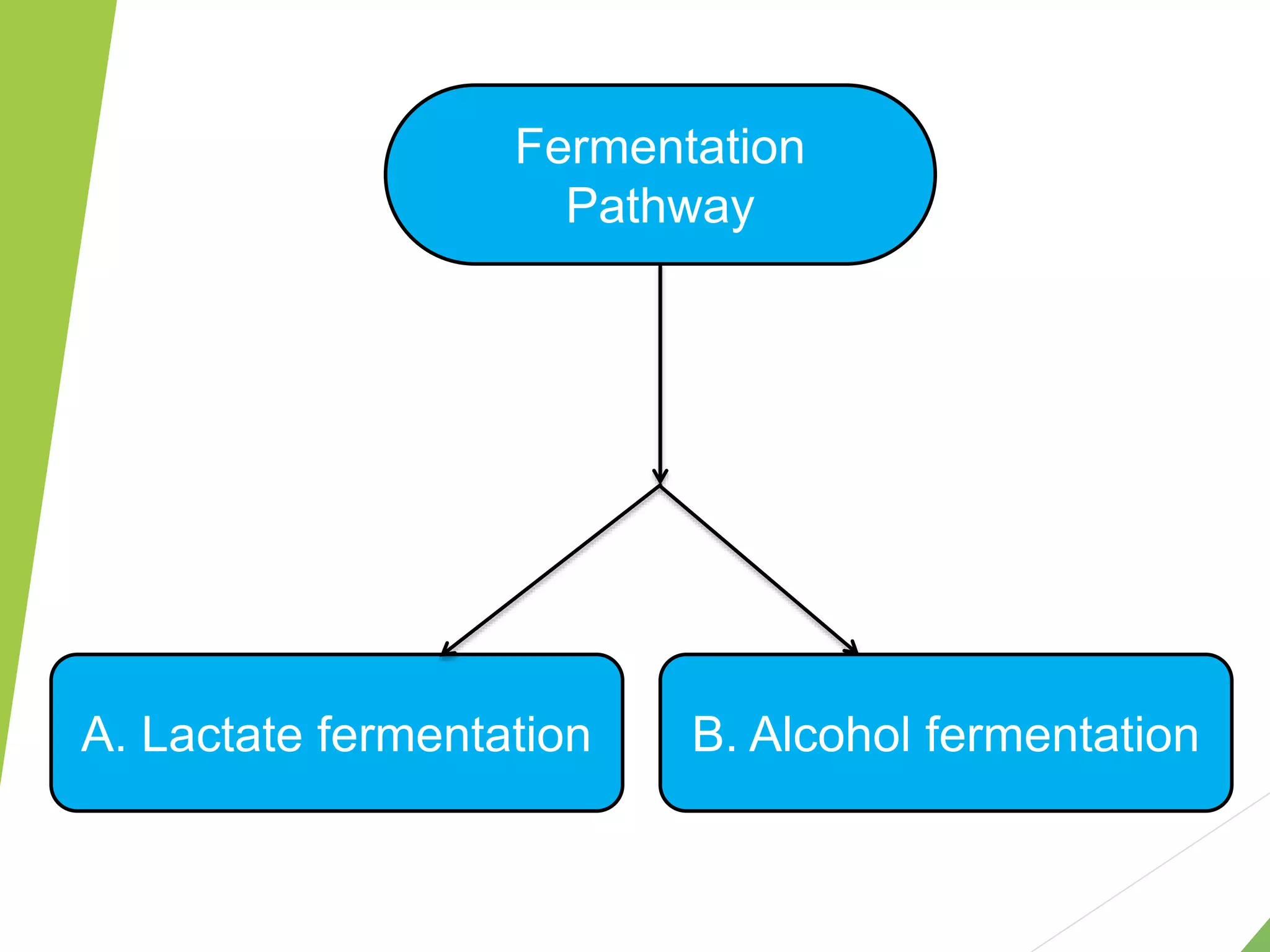
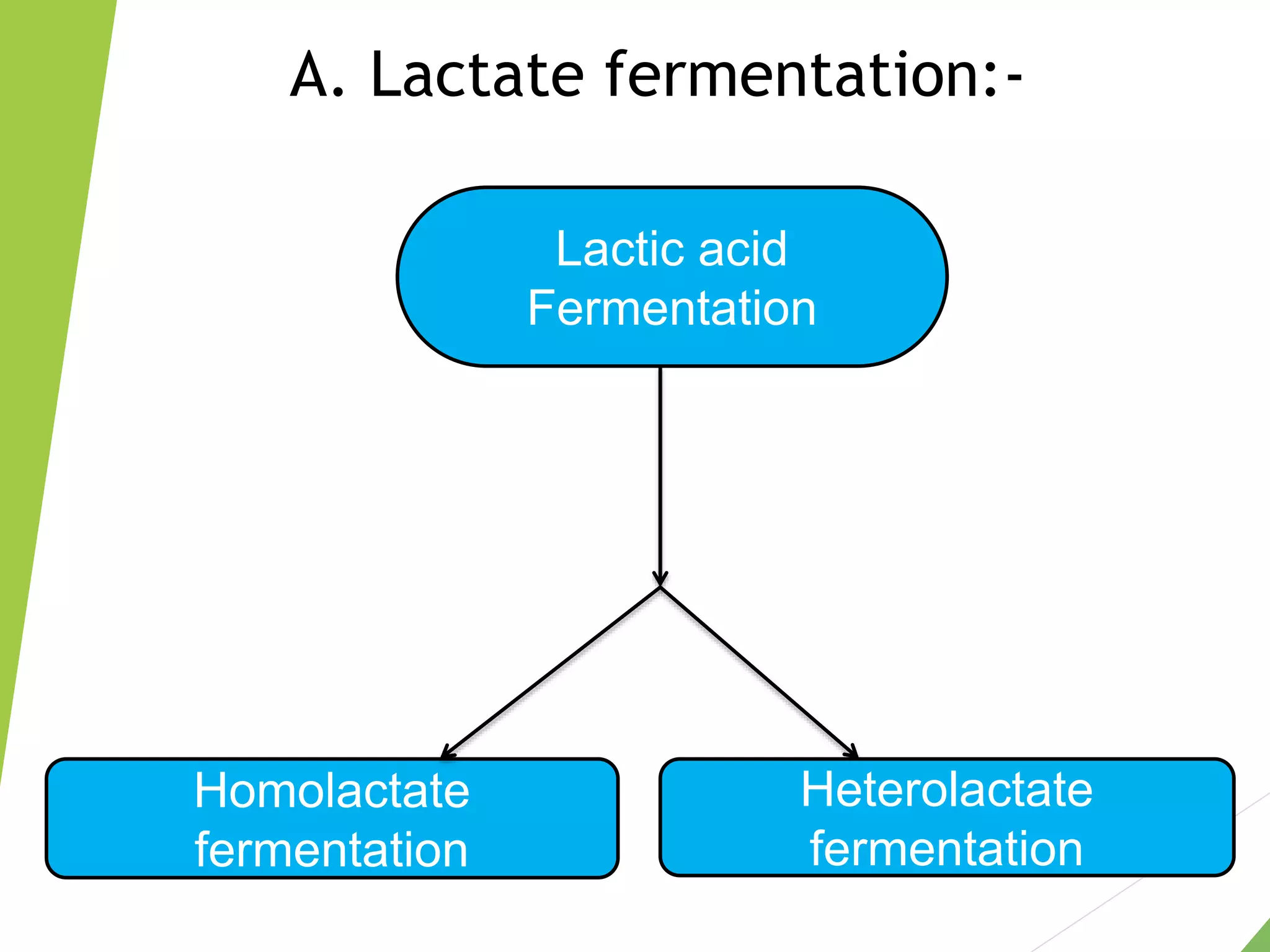
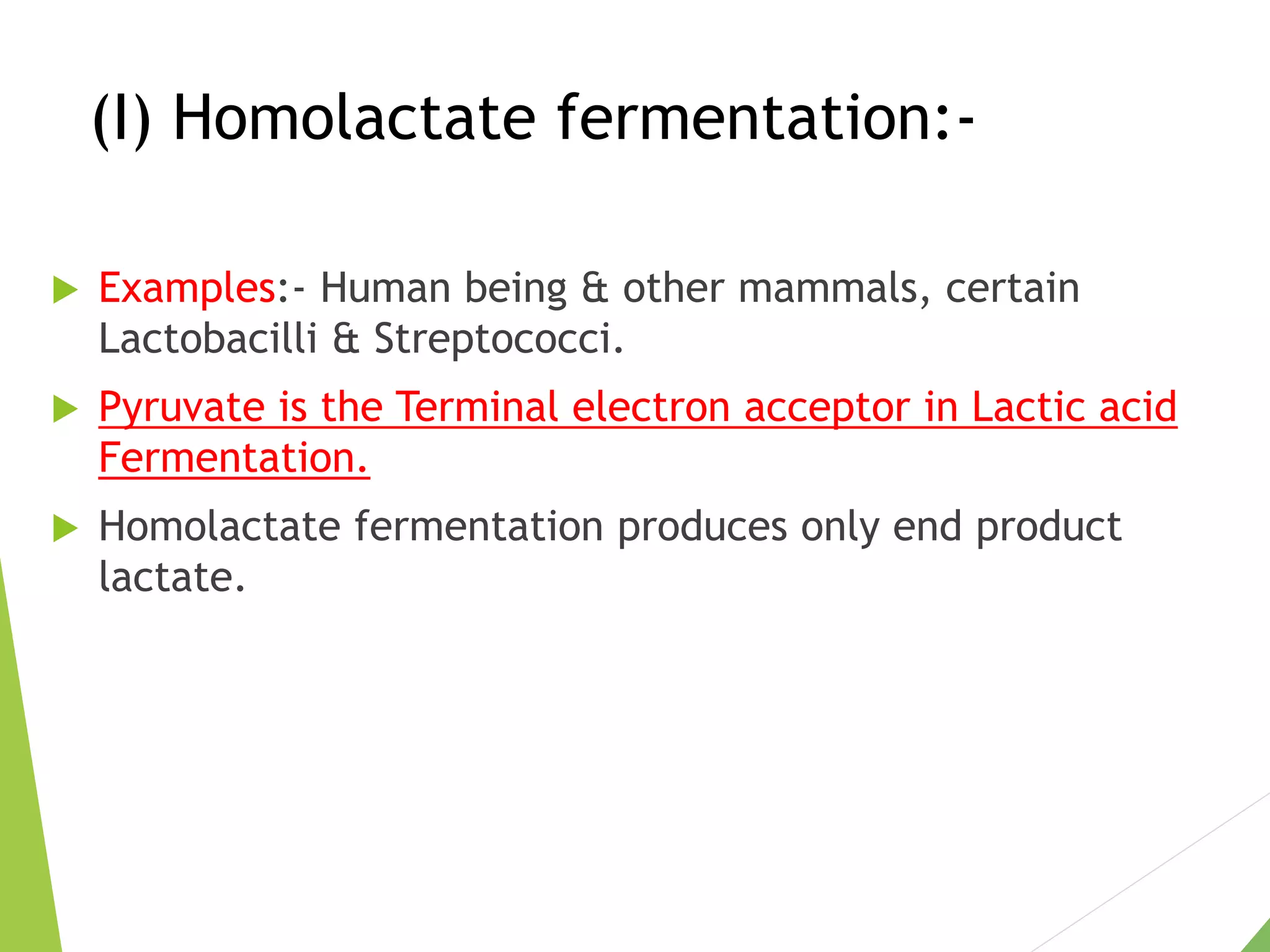
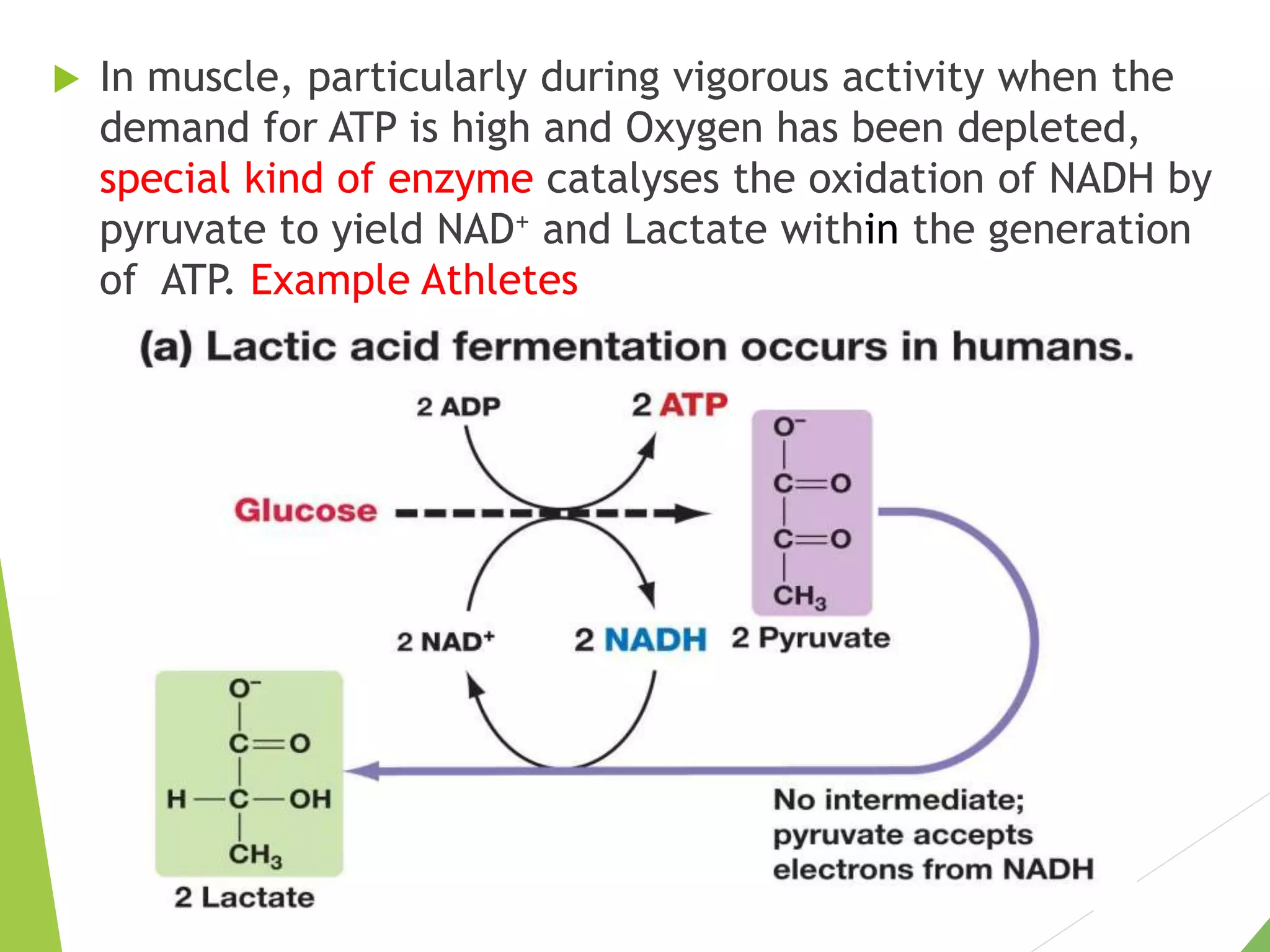
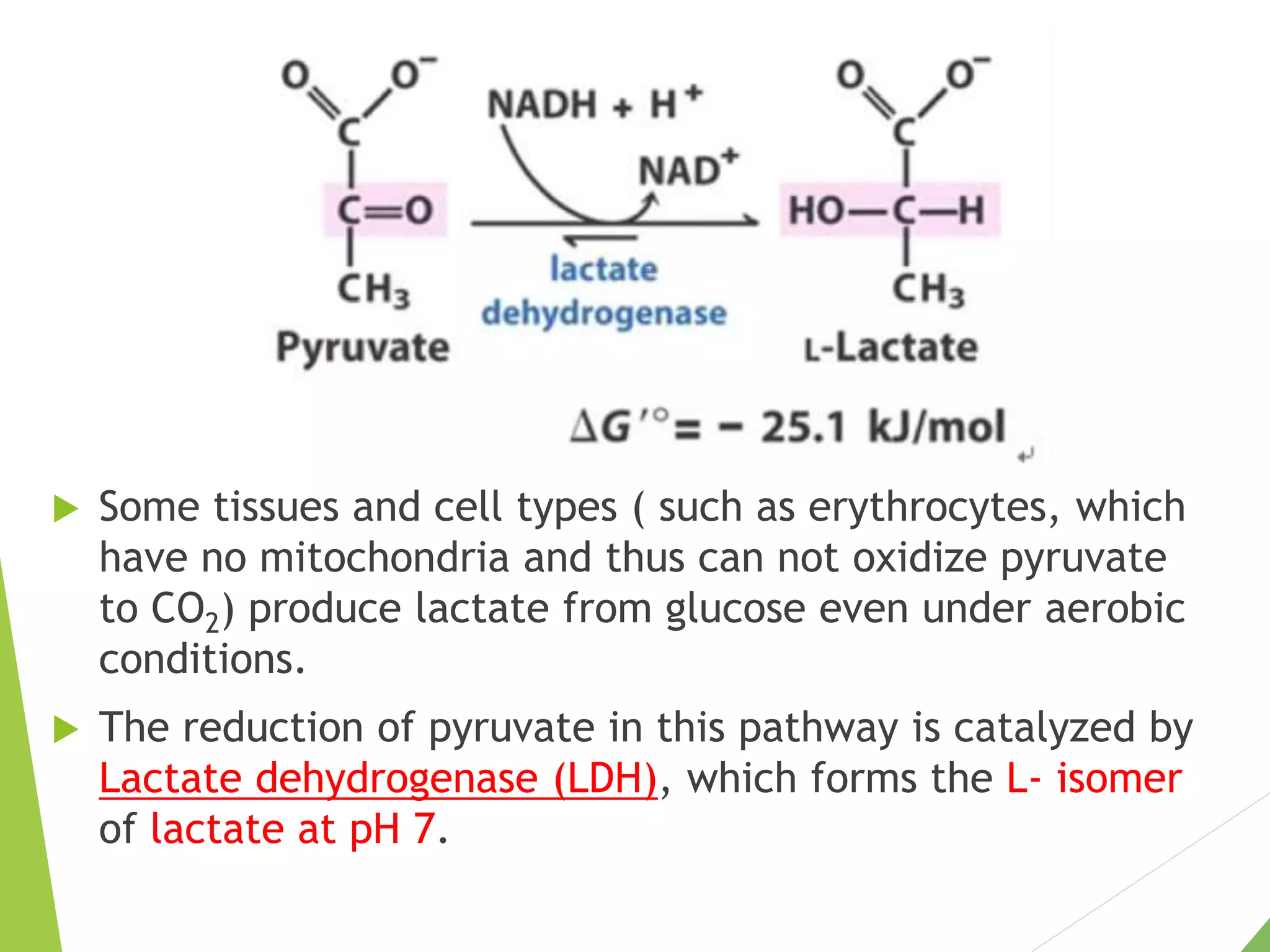
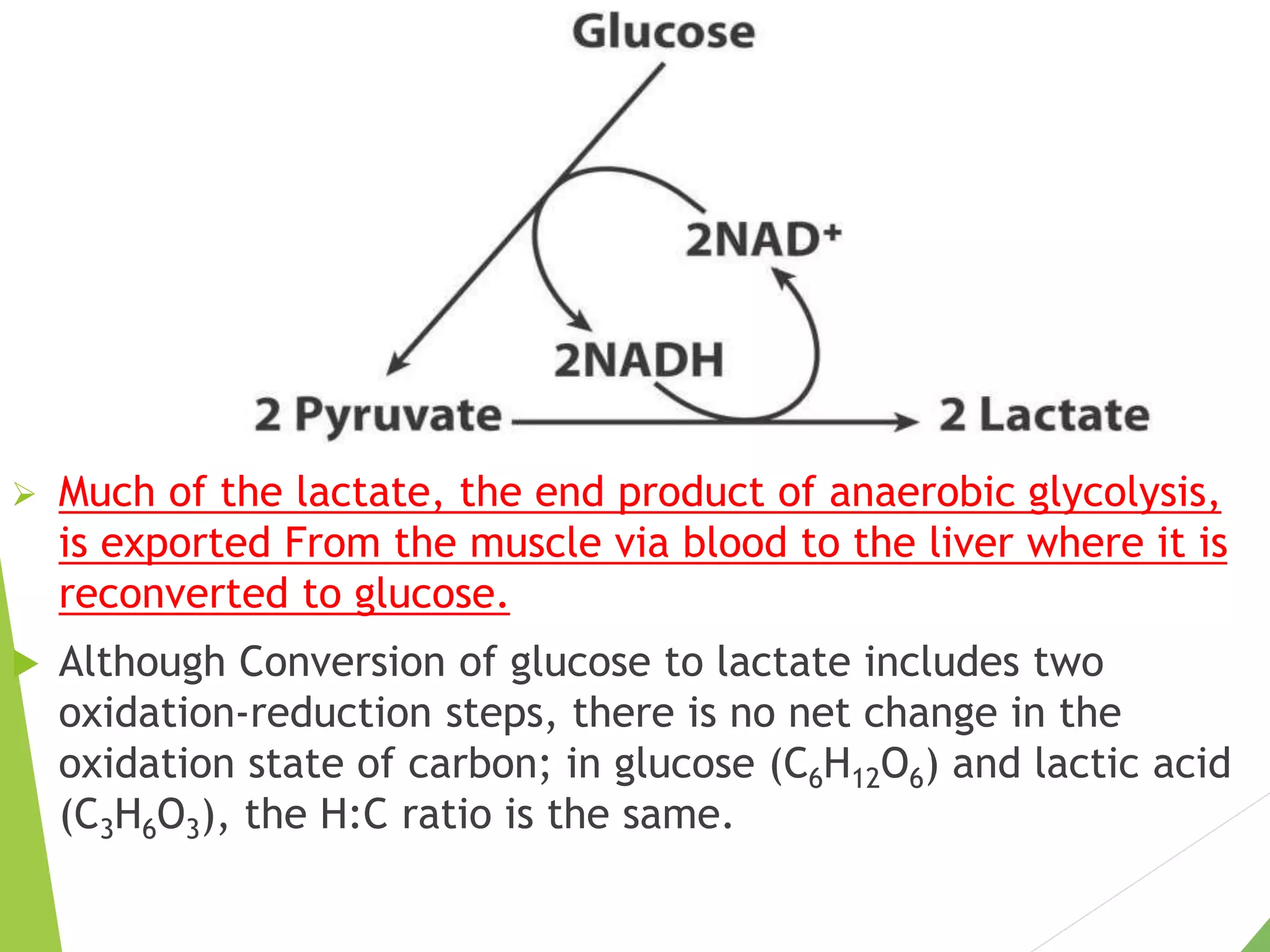
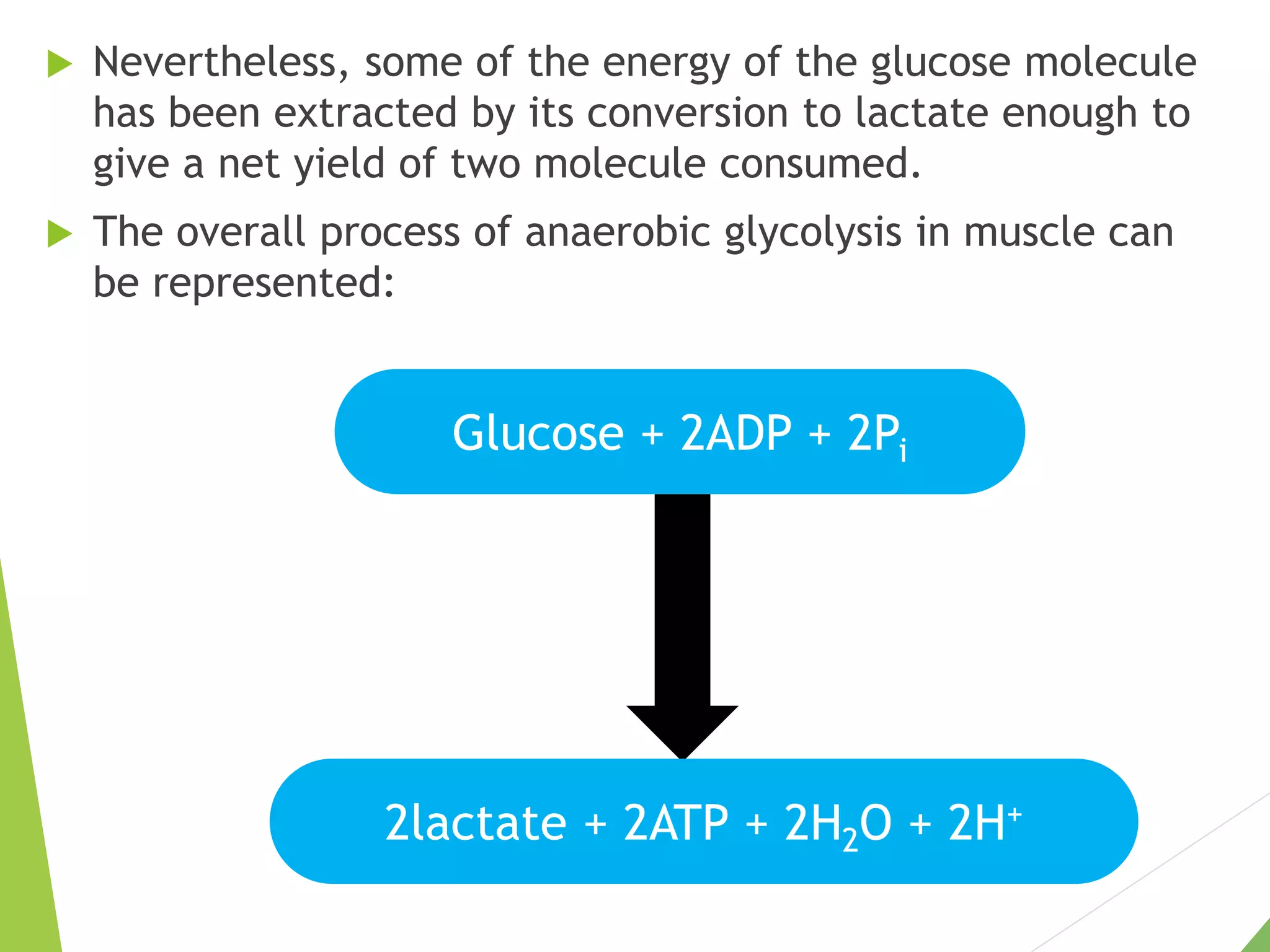
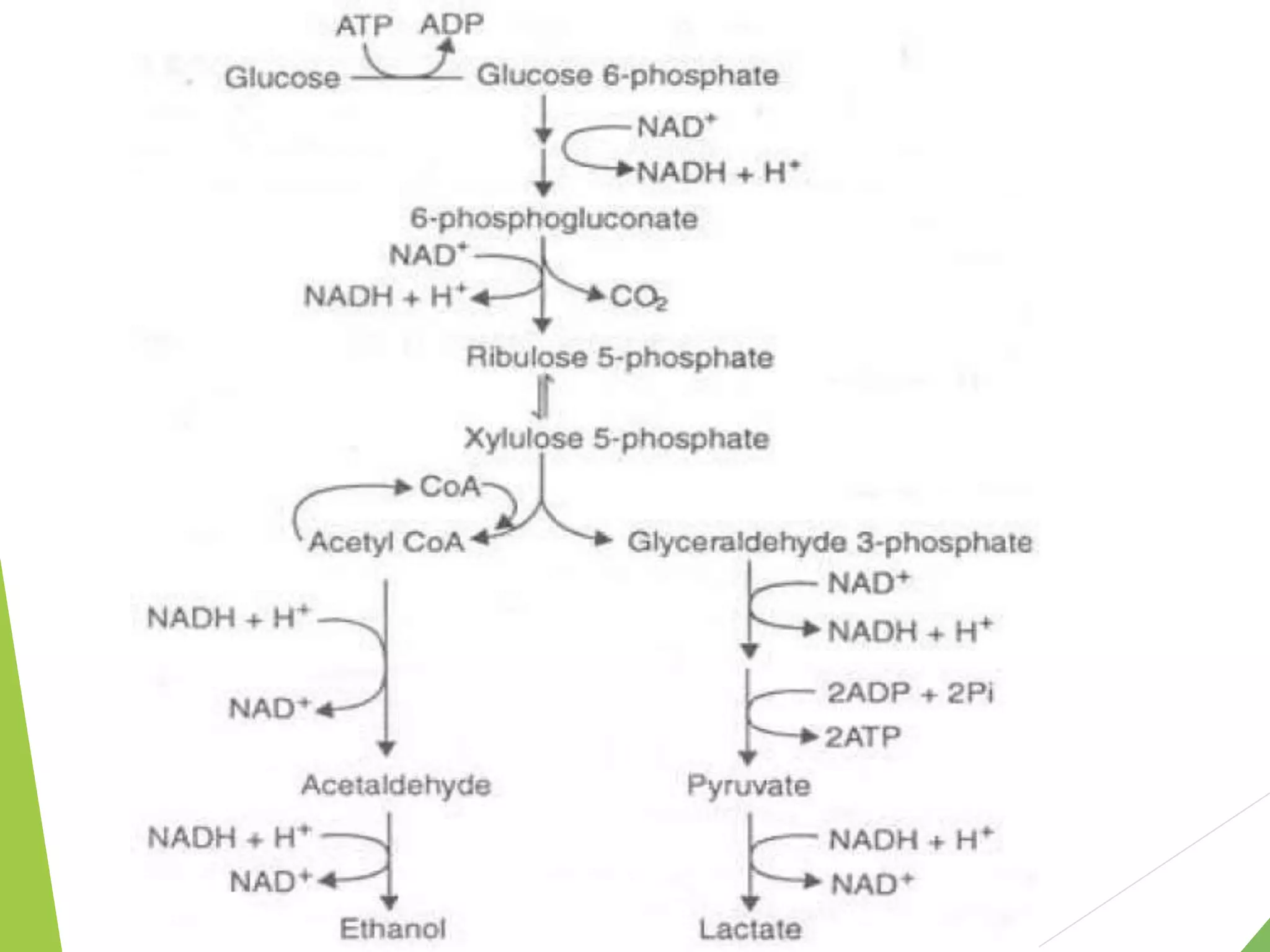
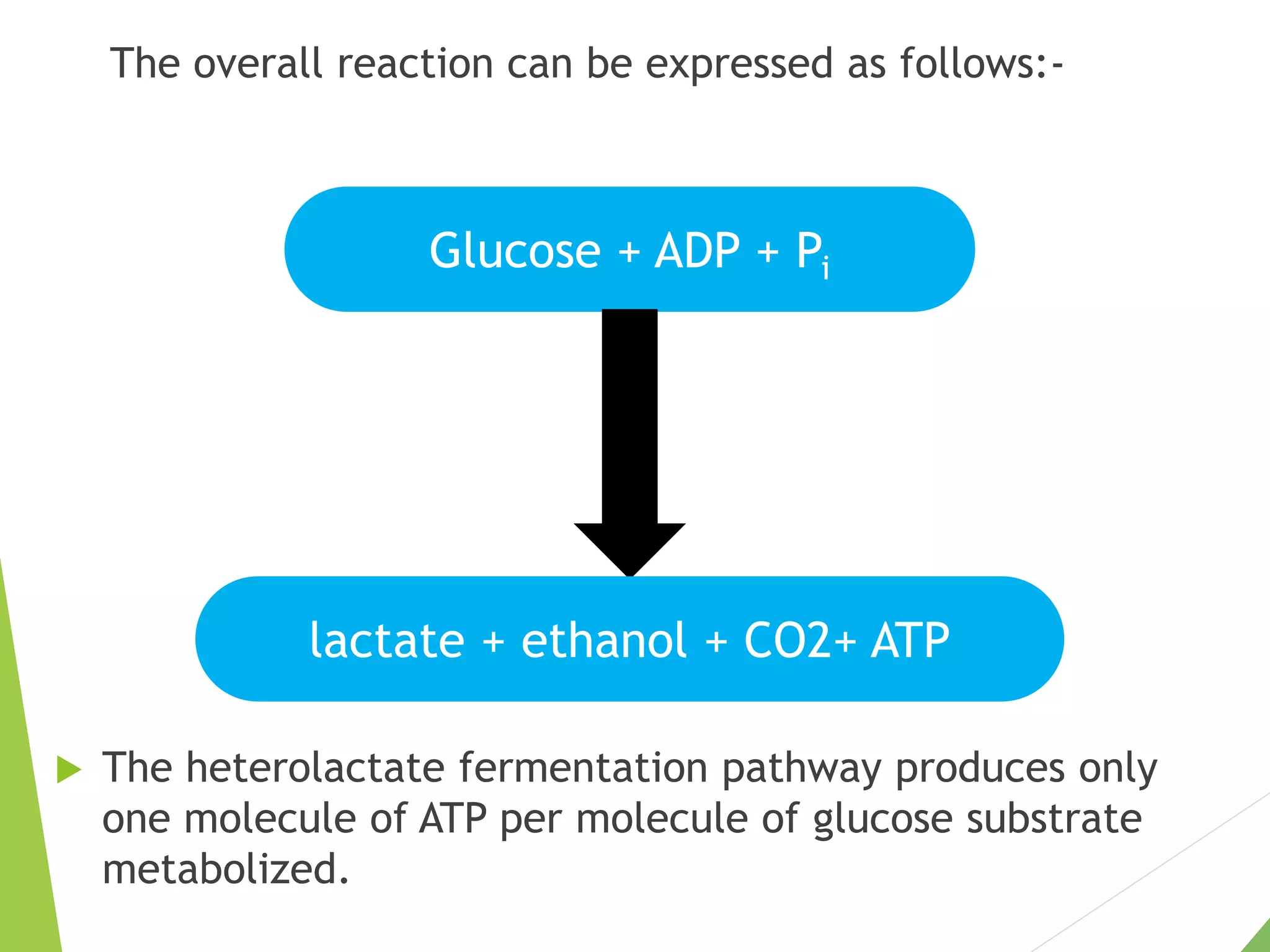
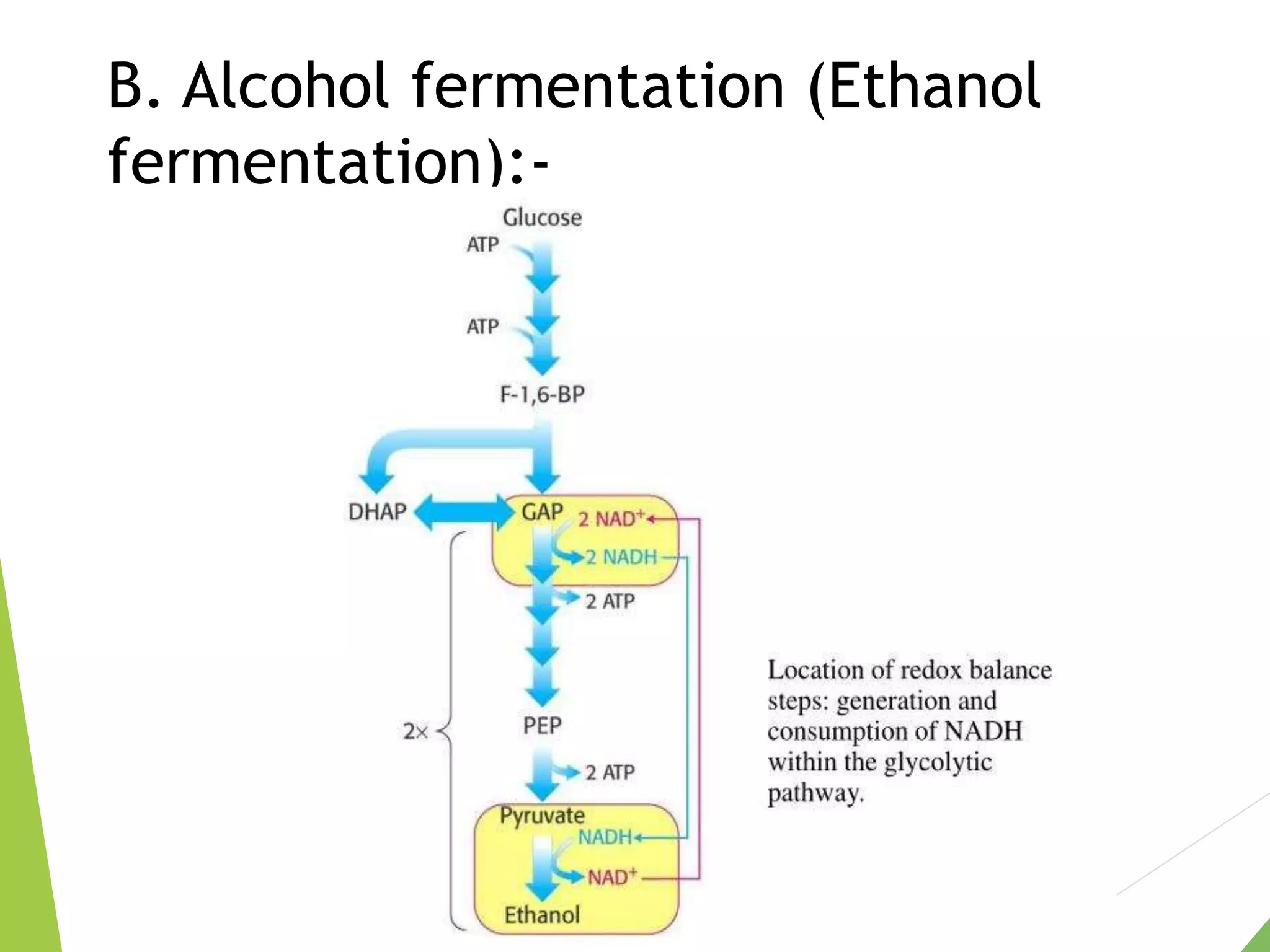
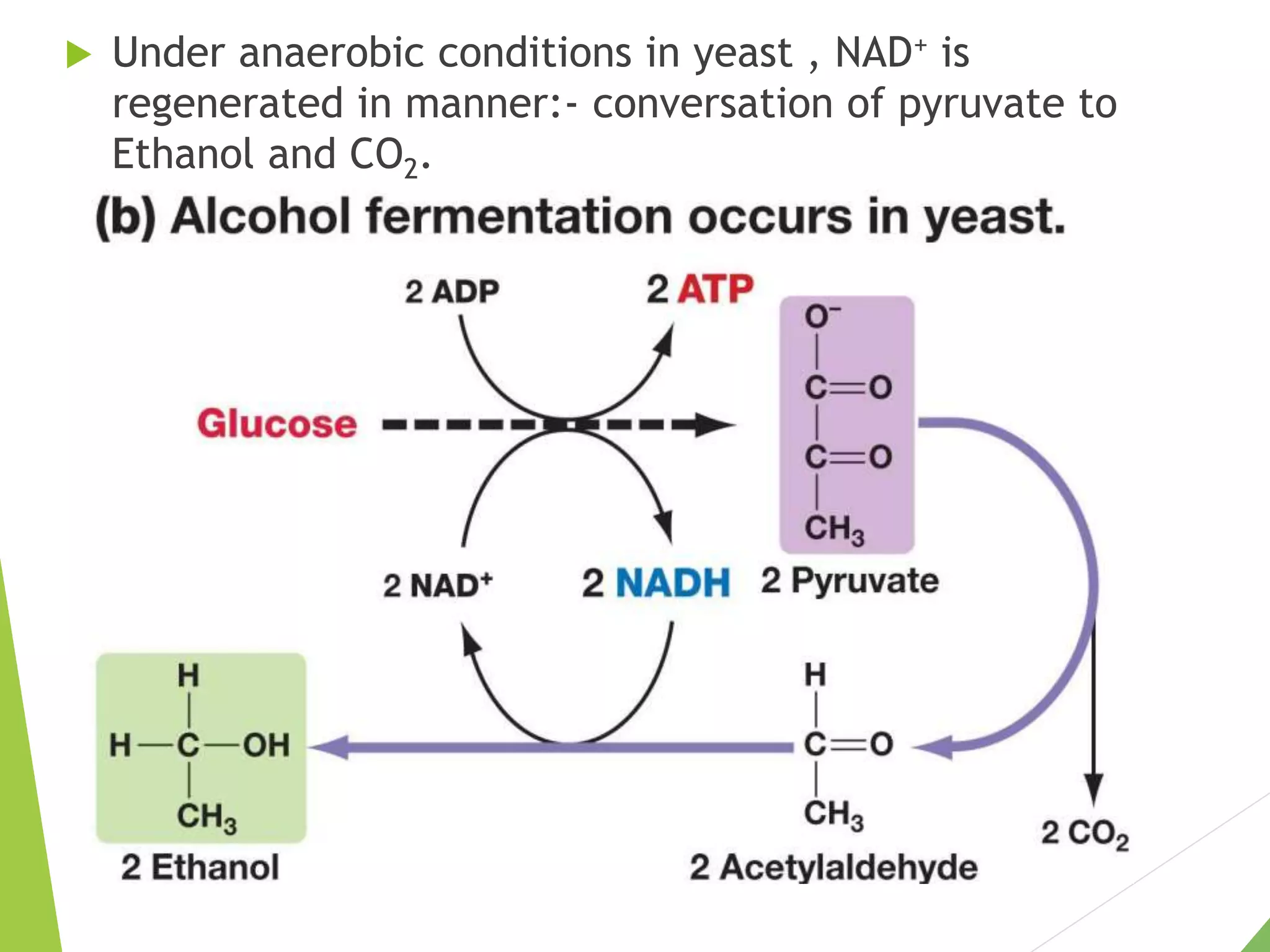
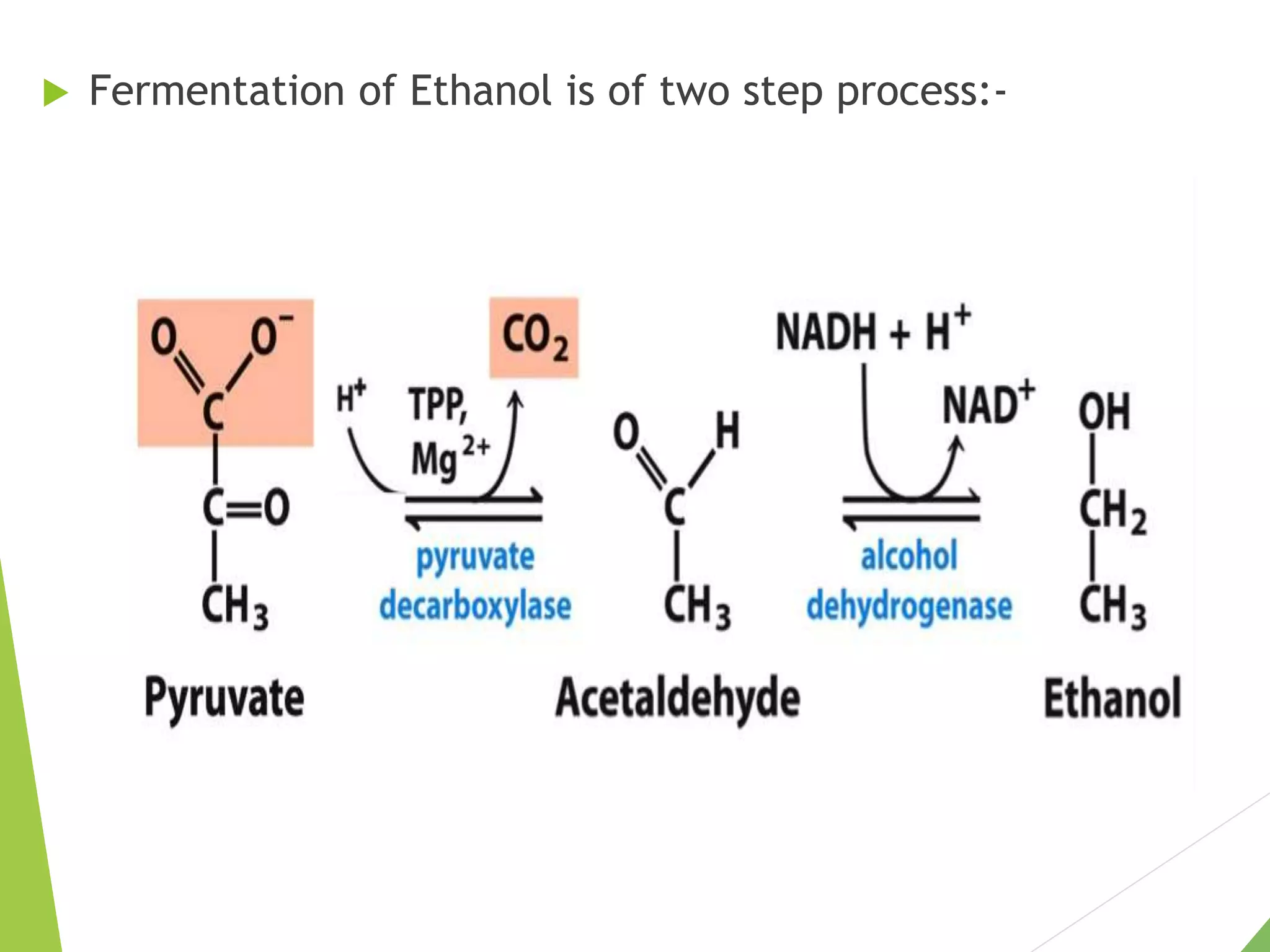
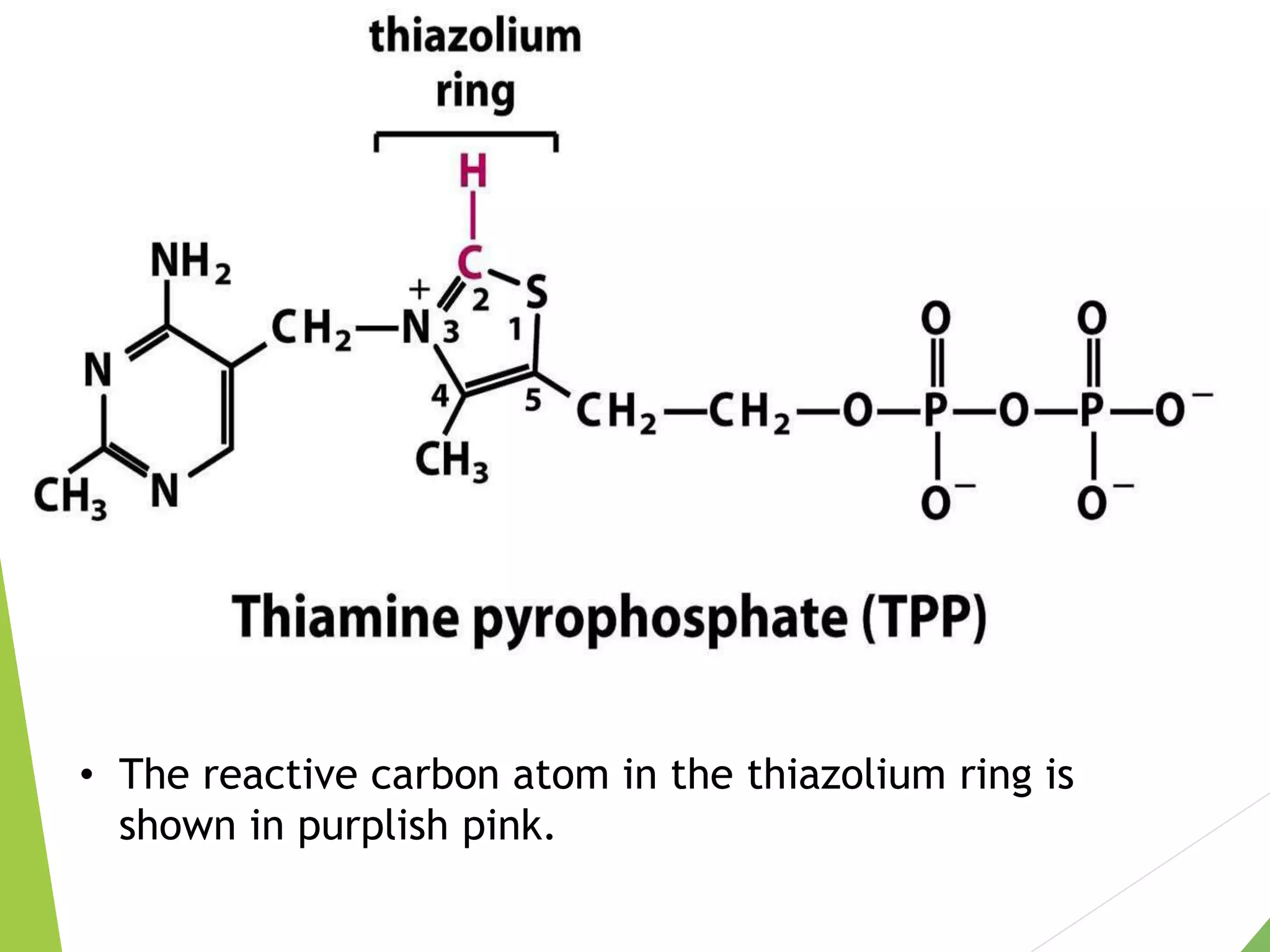
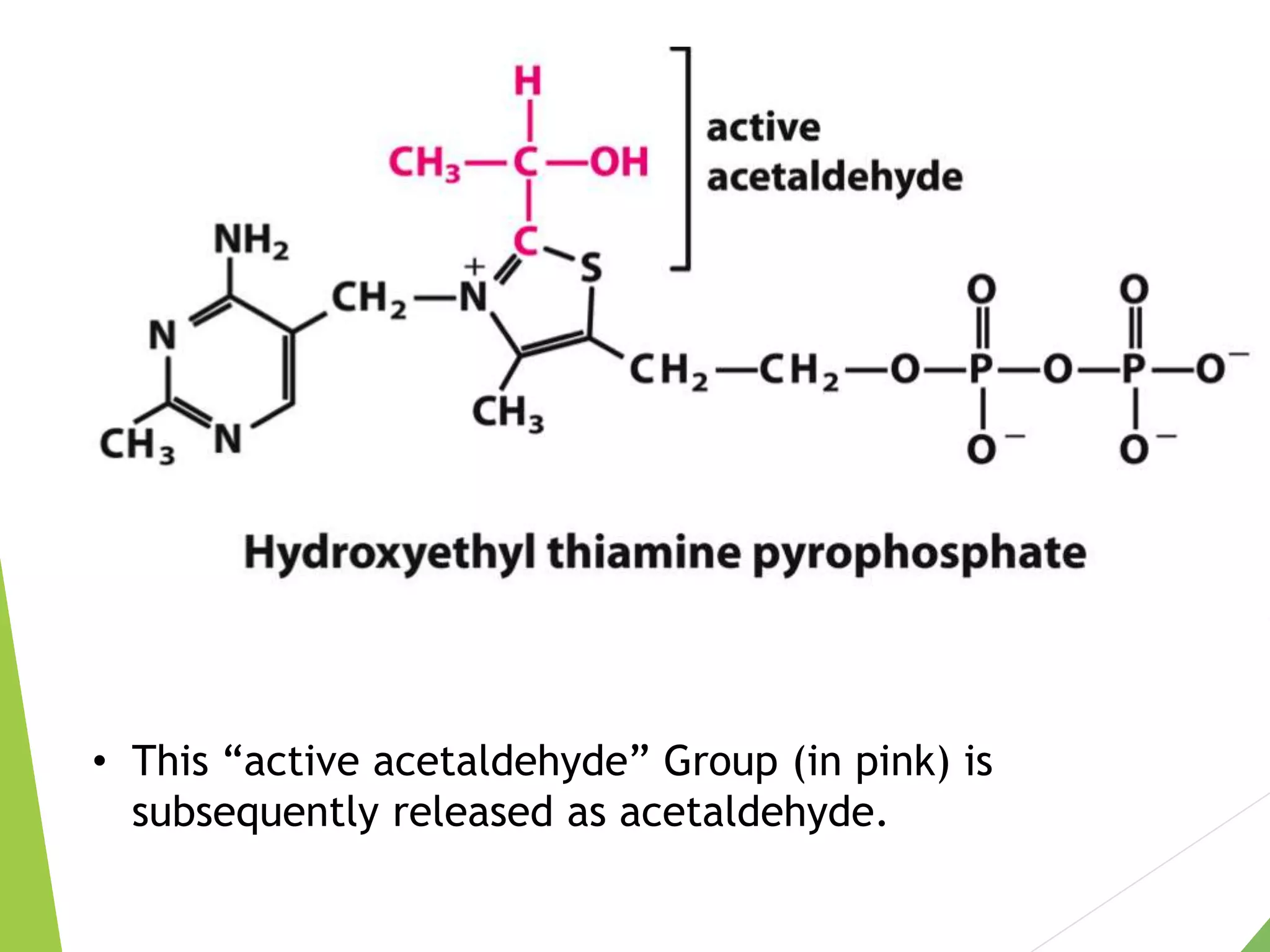
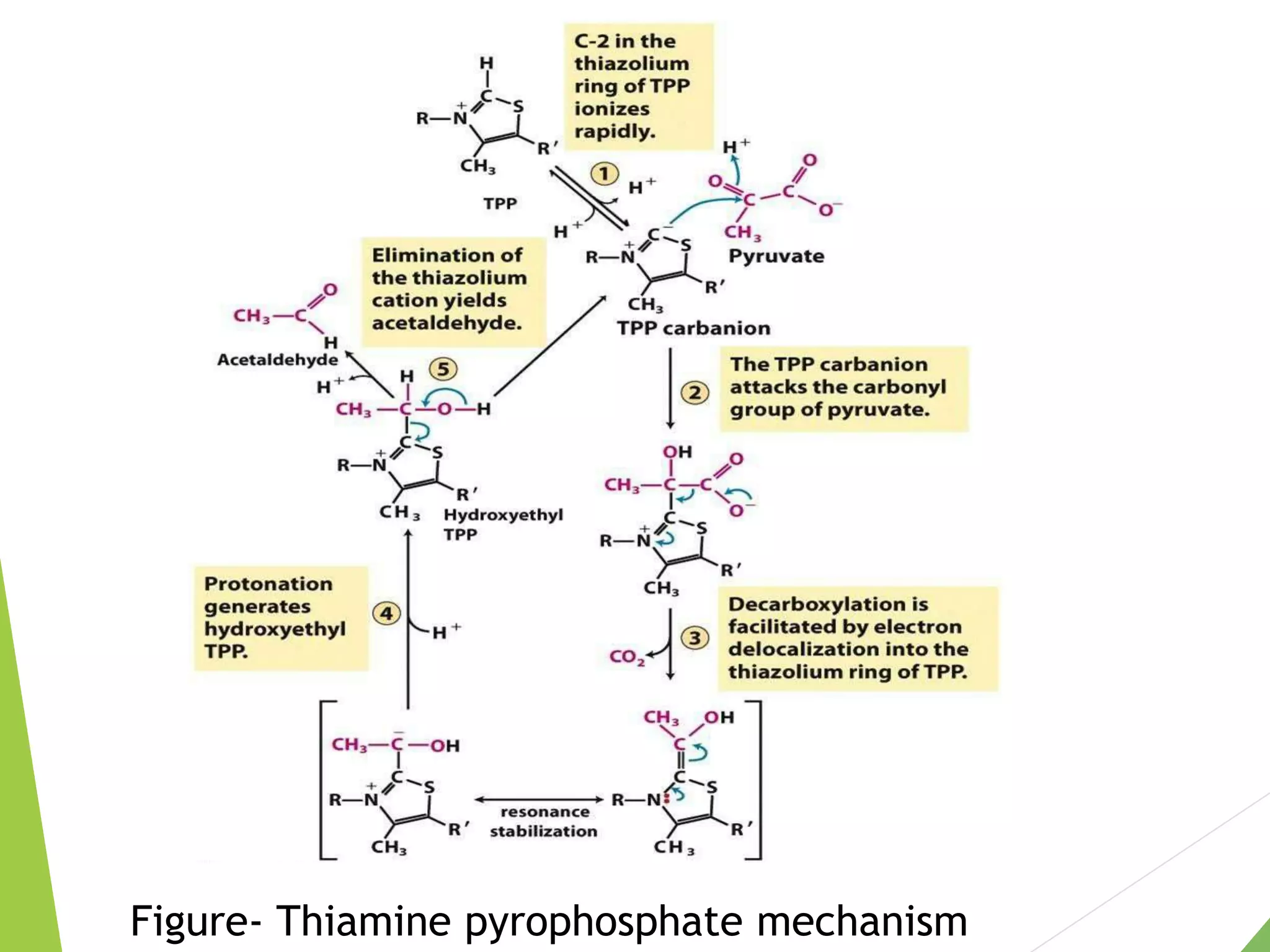
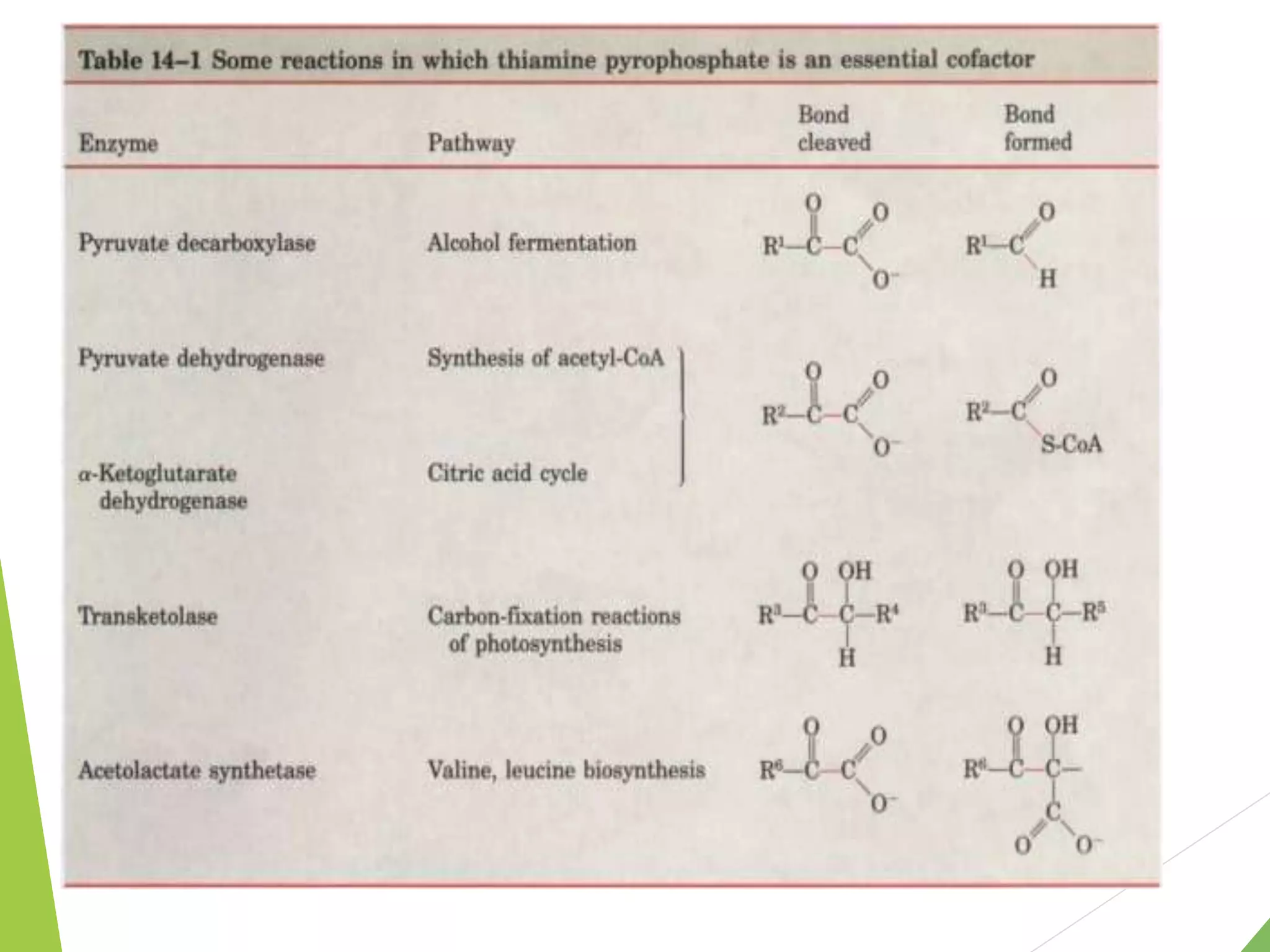
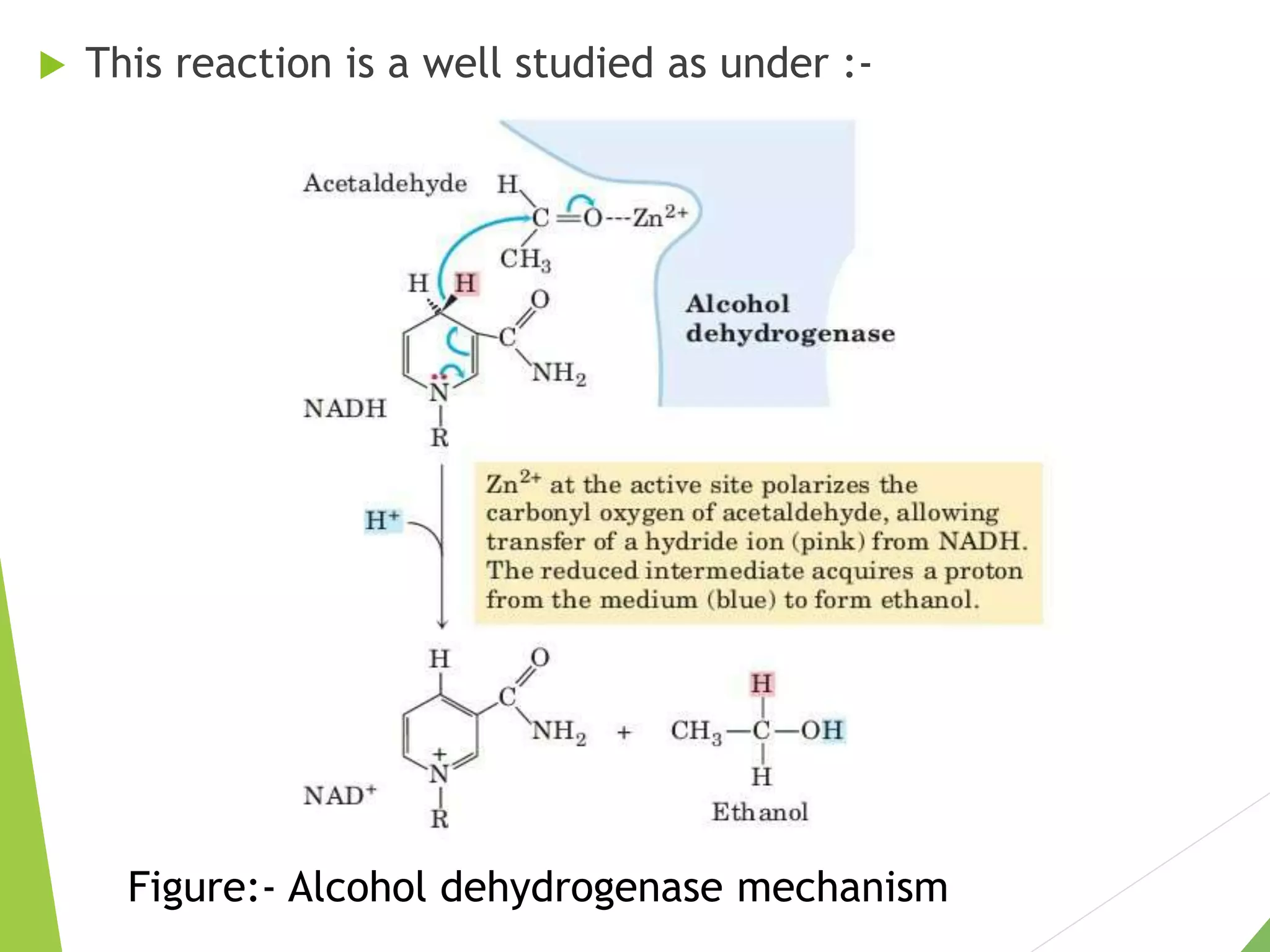
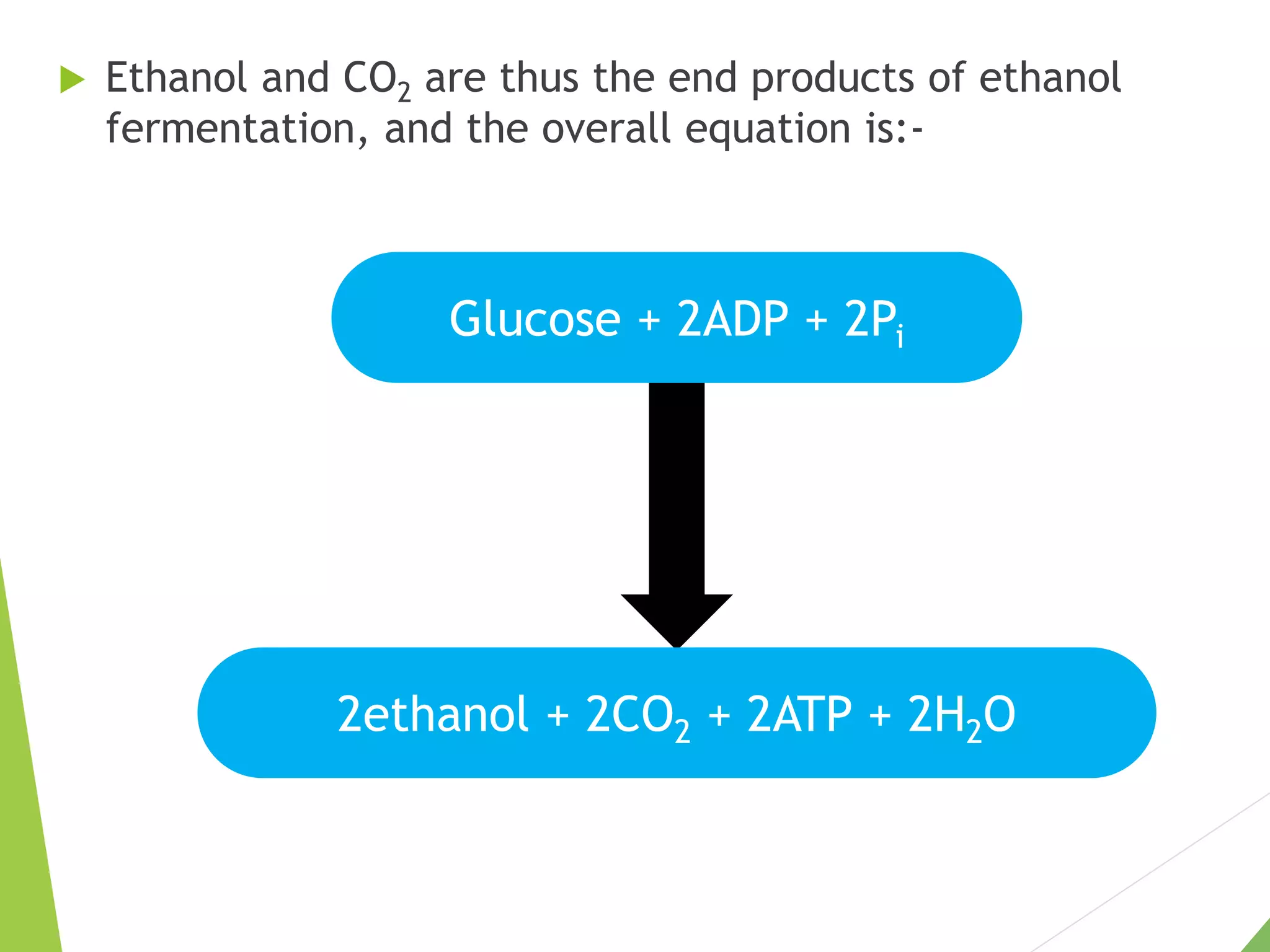
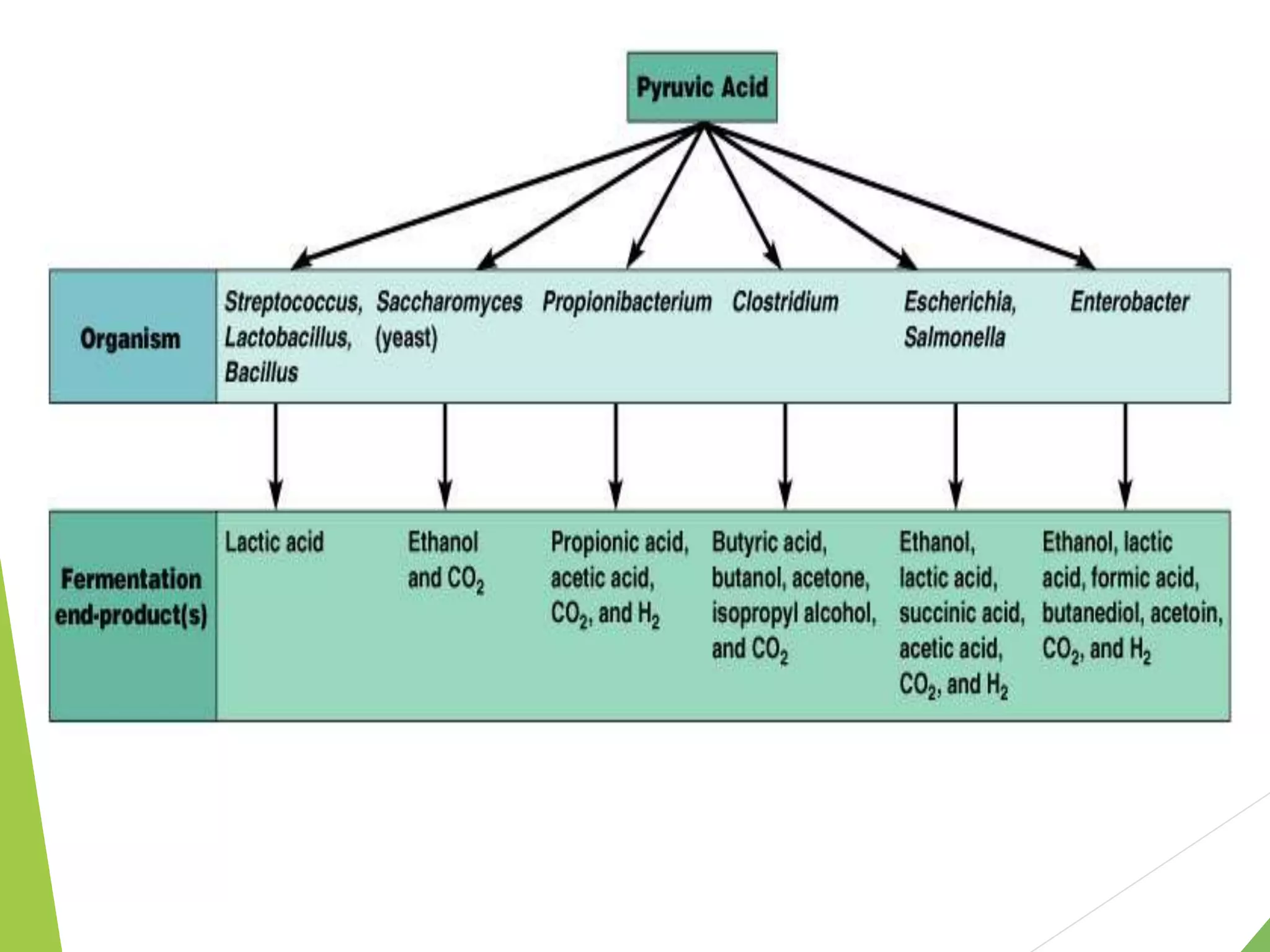
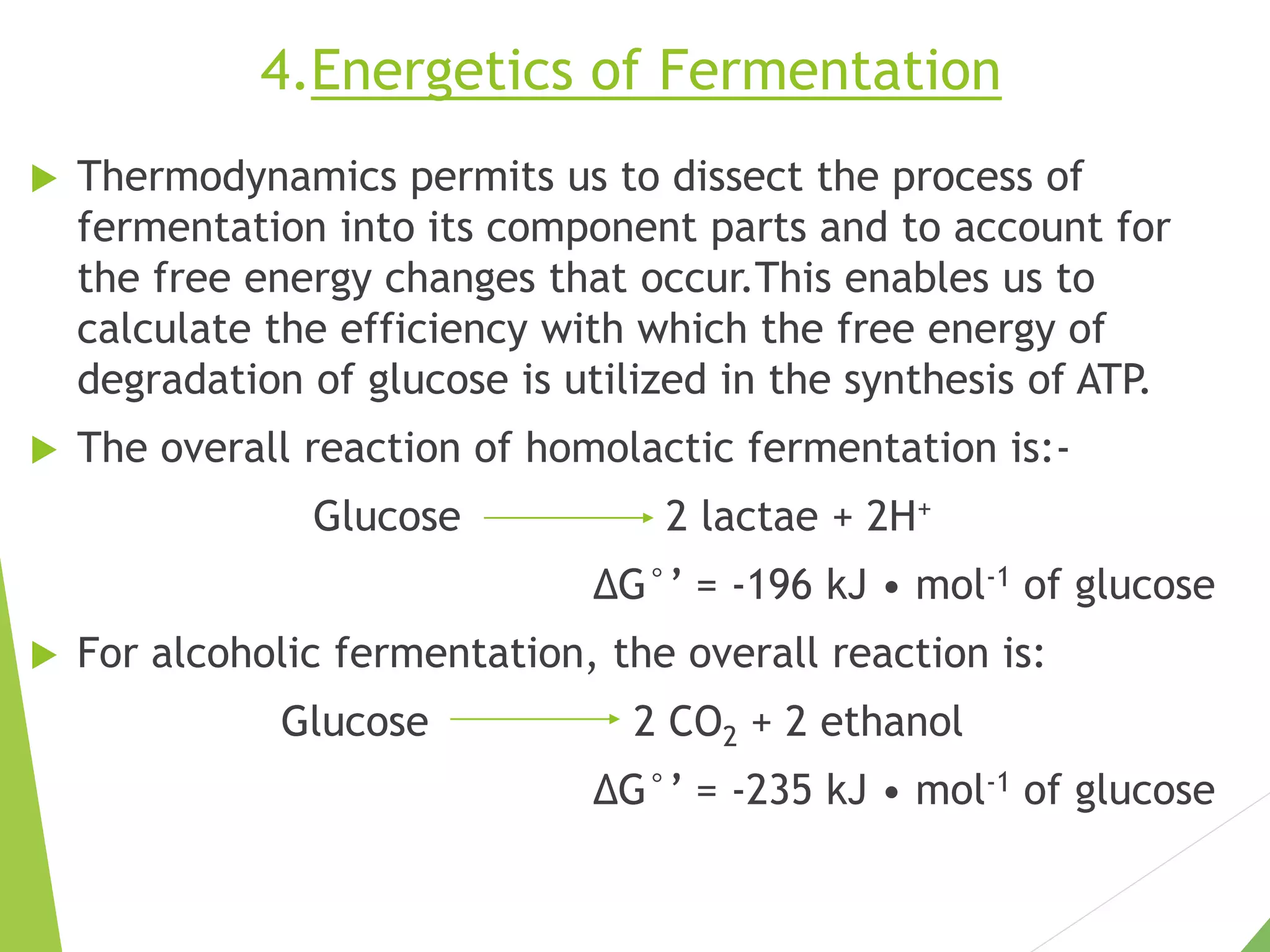
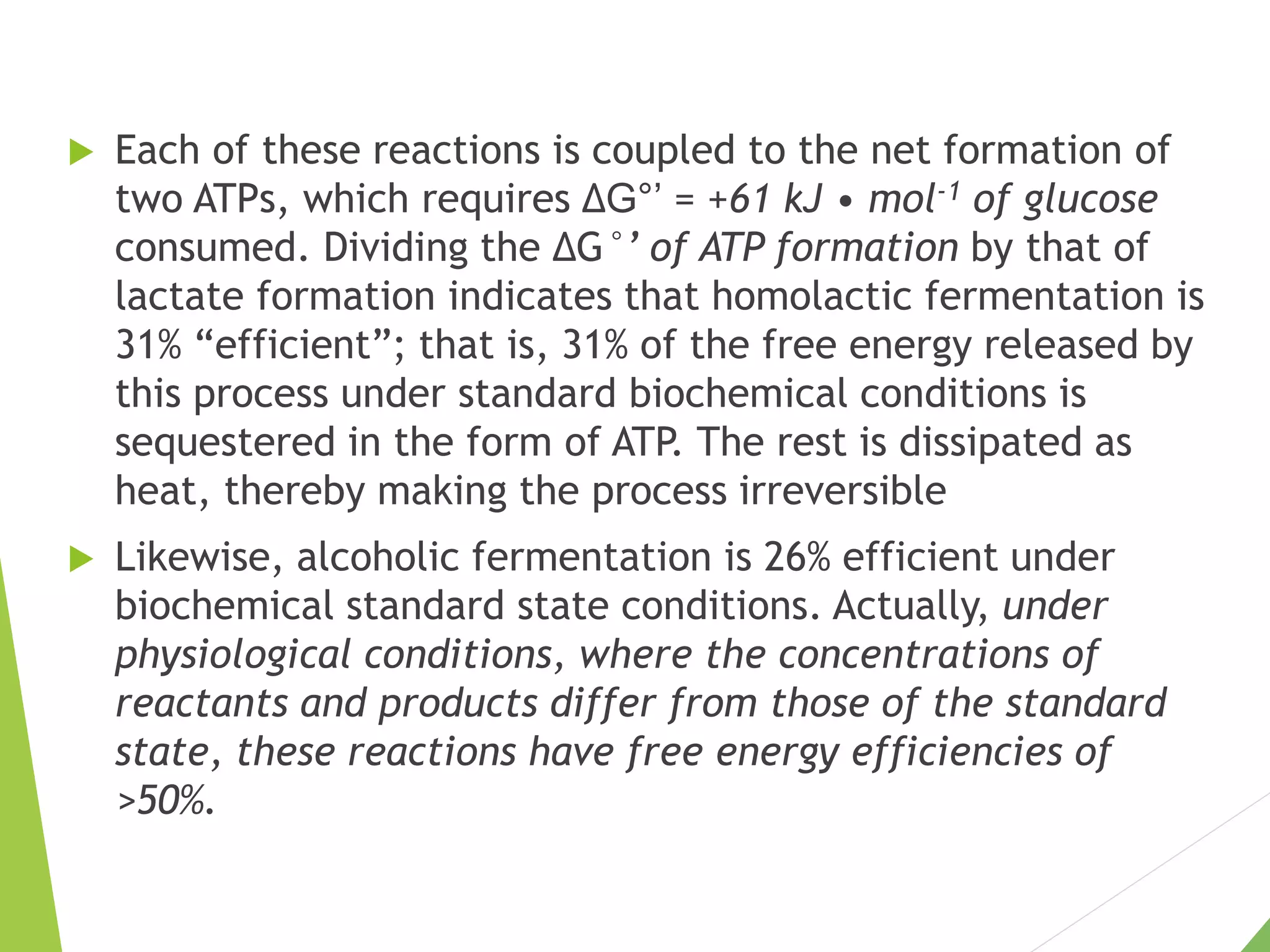
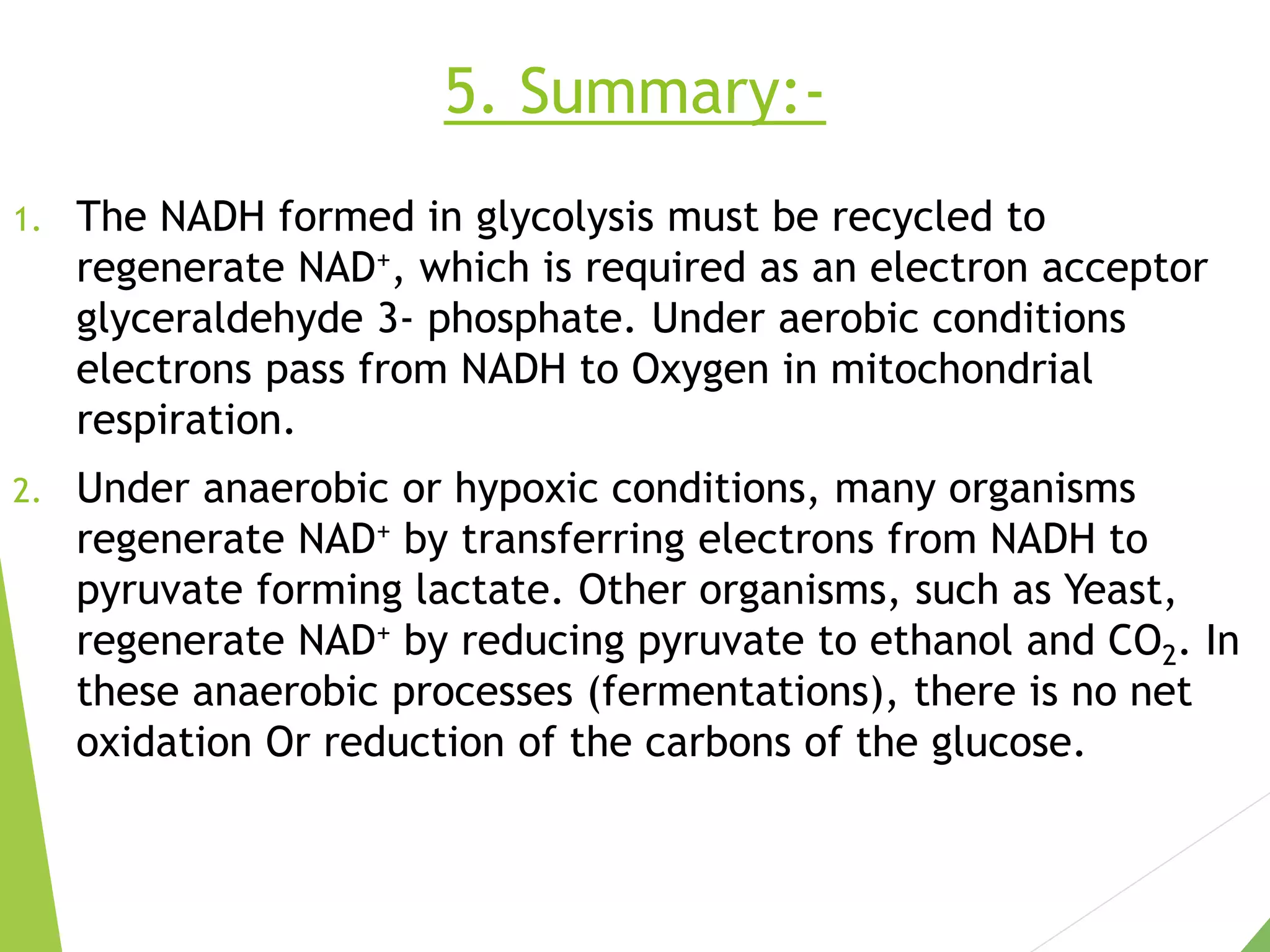
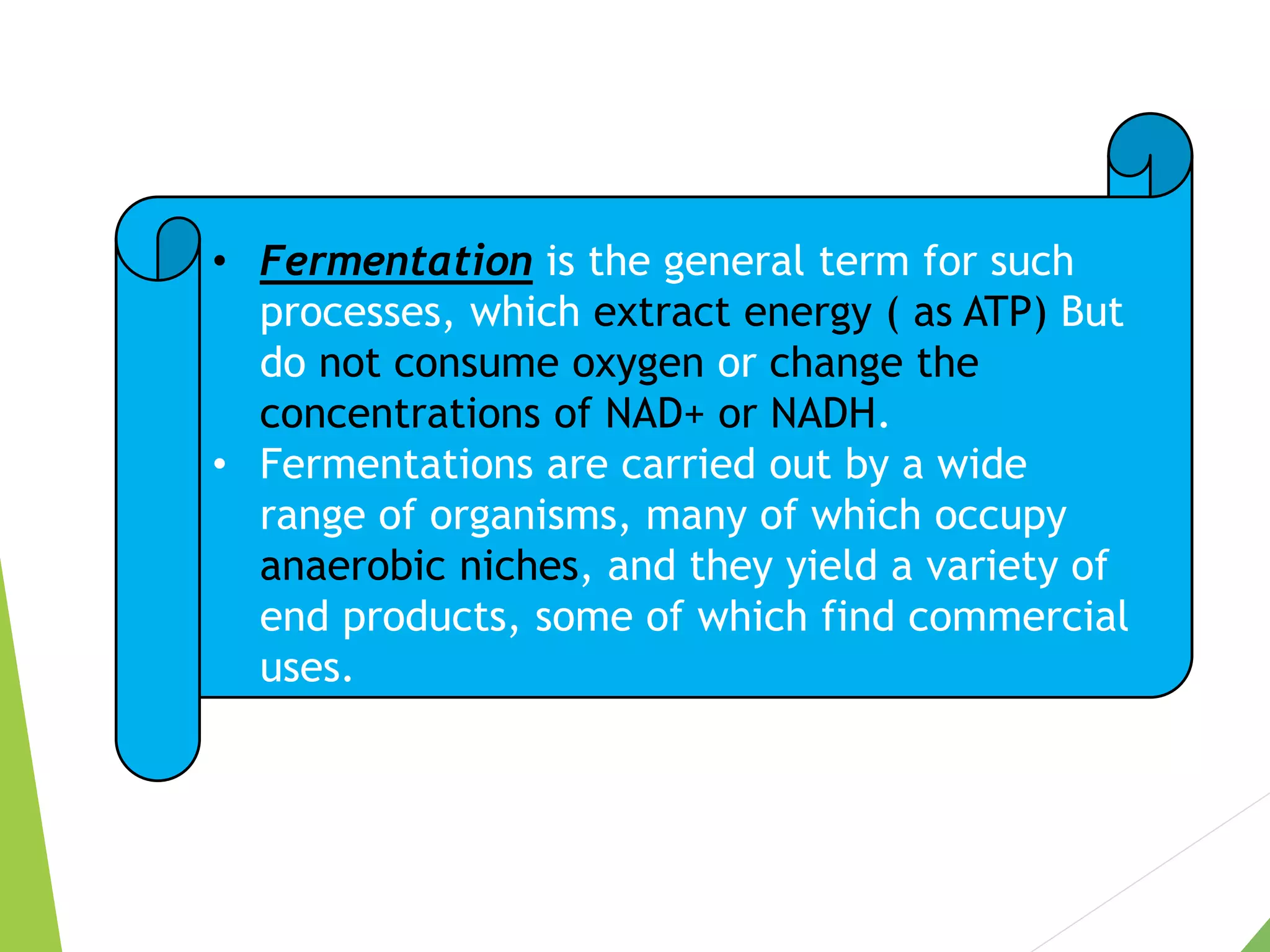
The document discusses the fate of pyruvate under anaerobic conditions, detailing processes like lactate and alcohol fermentation, where NAD+ is regenerated without oxygen. It highlights the roles of various microorganisms in fermentation, their effects on food production, and the thermodynamics of these processes. Additionally, it emphasizes that fermentation is energy-extracting without altering the concentrations of NAD+ or NADH.