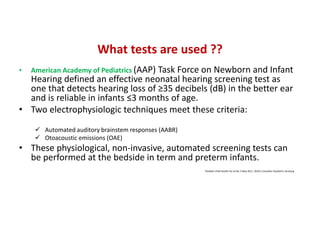
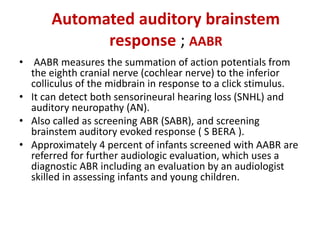
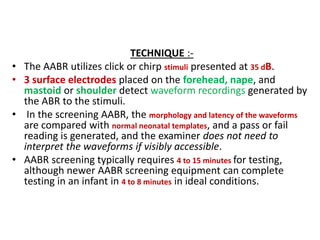
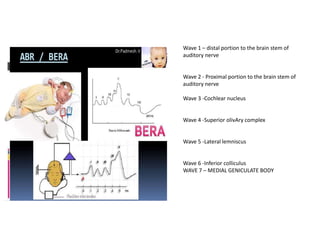
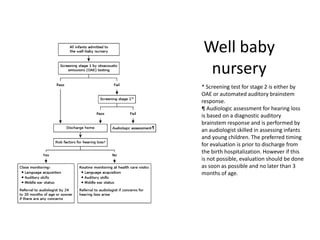
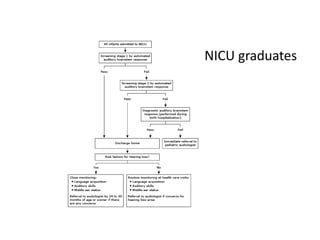
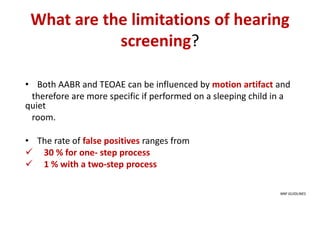
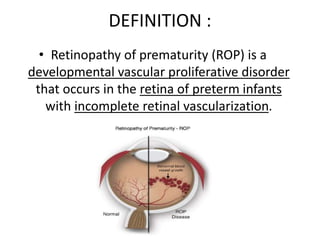
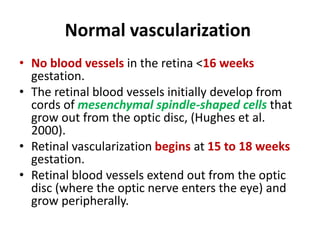
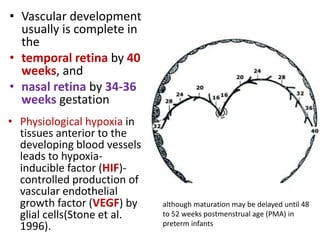
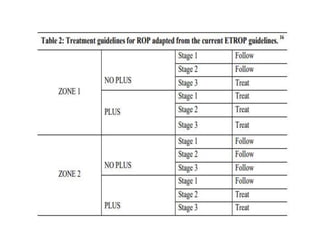
Retinopathy of prematurity (ROP) is a developmental vascular disorder of the retina that occurs in preterm infants. The retina is incompletely vascularized at birth for infants born before 30 weeks gestation. Premature birth interrupts normal retinal vascularization, exposing the retina to abnormal oxygen levels. This can cause vasoconstriction and arrest of blood vessel growth. Later, abnormal neovascularization may develop, potentially leading to retinal detachment and blindness if not treated. Screening guidelines recommend examinations starting at 4 weeks of age for infants born before 30 weeks, with treatment indicated for "threshold" or "pre-threshold" ROP. Laser photocoagulation is the primary treatment, which ablates the av


































![Syndromes associated with hearing
loss
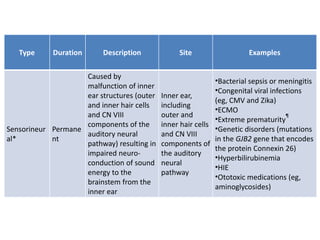
• Congenital Rubella
syndrome
• Usher syndrome
• Jervell and Lange-Nielsen
(JLN) syndrome
• Treacher-Collins syndrome
• Apert syndrome
• Alport syndrome
INDIAN PEDIATRICS 2 JUNE 04, 2017 [E-PUB AHEAD OF PRINT]CONSENSUS STATEMENT ON NEWBORN HEARING
SCREENING
• Neurofibromatosis syndrome
• Achondroplasia
• CHARGE syndrome
• Brachio Oto Renal syndrome
• Chudley McCullough syndrome
• Goldenhar syndrome.
INDIAN PEDIATRICS 2 JUNE 04, 2017 [E-PUB AHEAD OF PRINT]CONSENSUS
STATEMENT ON NEWBORN HEARING SCREENING](https://image.slidesharecdn.com/rophearing-220303194036/85/Rop-hearing-54-320.jpg)
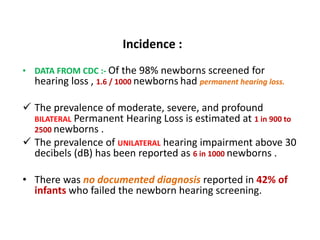
![• Screening newborns for hearing loss leads to Earlier Detection and
Intervention in patients with congenital hearing impairment.
• Early intervention can improve language acquisition and educational
achievement in affected patients.
• Vocabulary of a 3-year-old child with typical hearing which is 500-
900 words.
• Vocabulary of a 3-year-old child with hearing impairment if :-
If remediated at birth is 300-700 words
If re-mediated at 6 months is 150-300 words
If remediated at 2 years is 0-50 words, respectively
INDIAN PEDIATRICS 2 JUNE 04, 2017 [E-PUB AHEAD OF PRINT]CONSENSUS STATEMENT ON NEWBORN HEARING SCREENING
Screening guidelines](https://image.slidesharecdn.com/rophearing-220303194036/85/Rop-hearing-55-320.jpg)