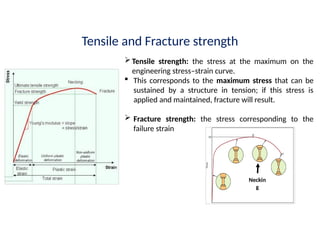
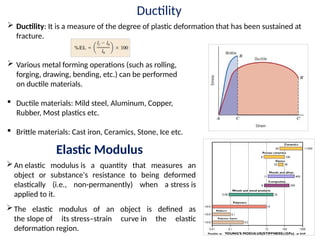
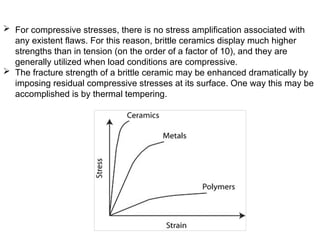
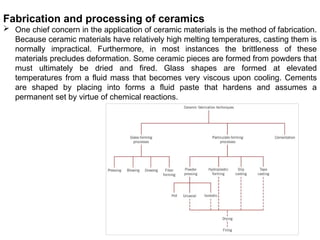
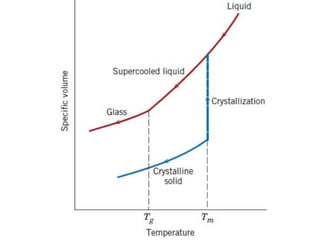
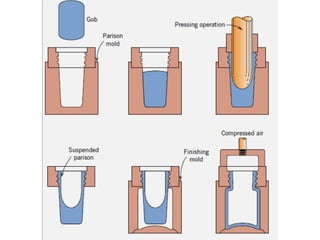
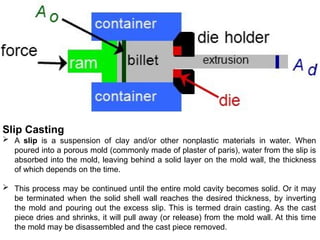
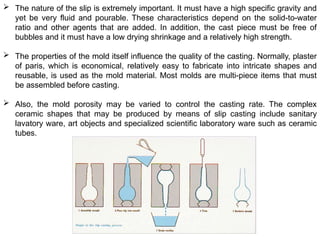
The document provides an extensive overview of ceramic materials, discussing their properties, structures, bonding characteristics, and types of defects. It describes various crystal structures, fabrication methods, and the mechanical properties of ceramics, including stress-strain behavior, fracture characteristics, and processing techniques. Additionally, the document explains how ceramics can be shaped and thermally treated to enhance their performance, focusing on key processes such as hydroplastic forming and slip casting.




















![Reference:
[1] Materials Science and Engineering An Intriduction, 8th
edition, John
Wiley & Sons, Inc. by William D. Callister, Jr. and David G. Rethwisch](https://image.slidesharecdn.com/unit1-240907113406-c7ad26f9/85/Ceramics-Properties-Fabrication-and-Applications-41-320.jpg)