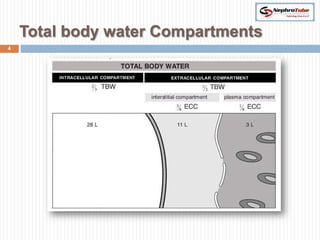
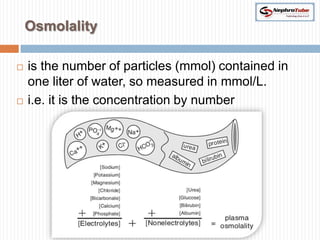
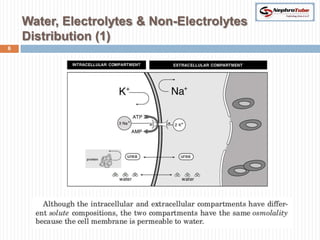
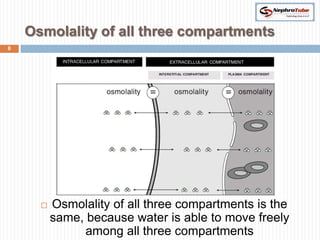
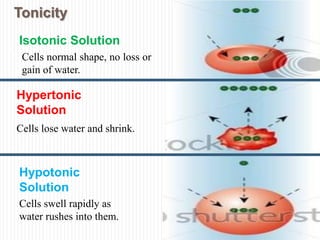
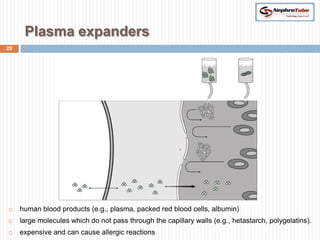
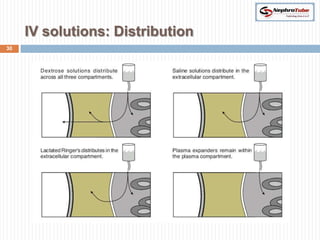
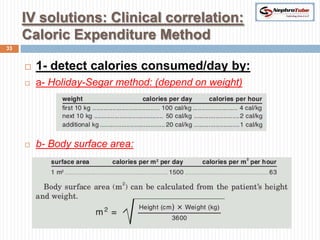
The document covers renal physiology and the application of intravenous (IV) fluids in clinical settings, detailing the body water compartments and the distribution of electrolytes. It discusses various forms of IV fluids, including dextrose, saline, and lactated Ringer's, alongside their tonicity, uses, and clinical correlations. Additionally, it addresses the caloric expenditure method for determining maintenance IV fluid requirements in patients unable to eat or drink.