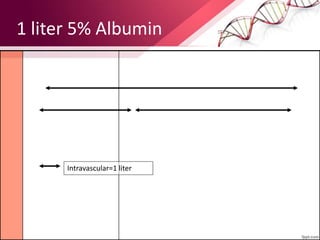
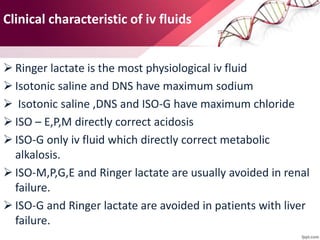
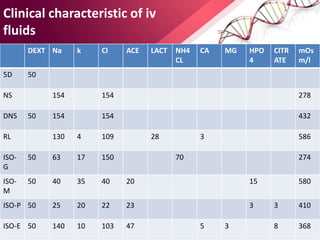
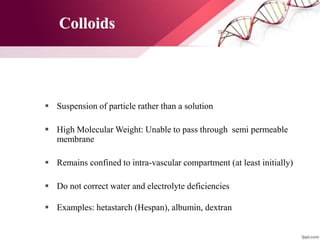
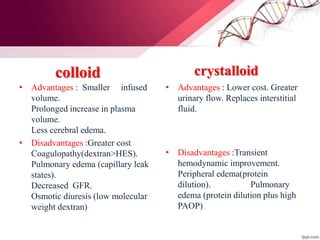
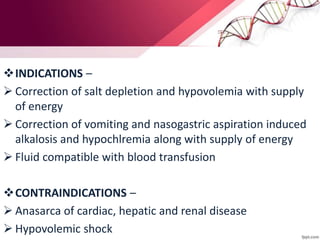
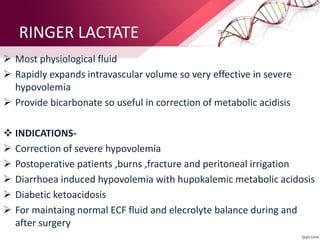
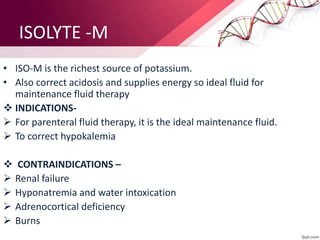
1. Ringers lactate is the most physiological intravenous fluid. Isotonic saline and DNS have the highest sodium content while isotonic saline, DNS, and ISO-G have the highest chloride levels.
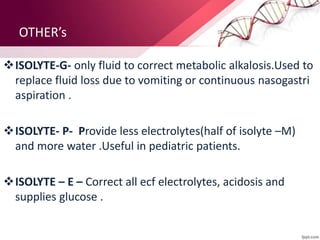
2. Isolyte-G is the only intravenous fluid that can directly correct metabolic alkalosis. Isolyte-M, P, G, and E along with Ringer's lactate should generally be avoided in patients with renal failure.
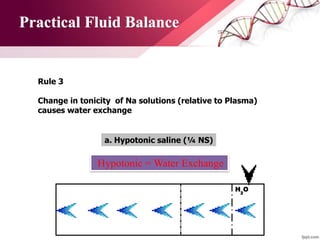
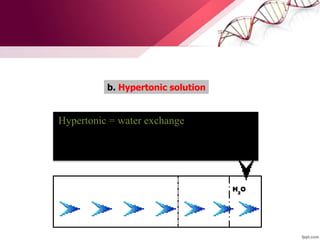
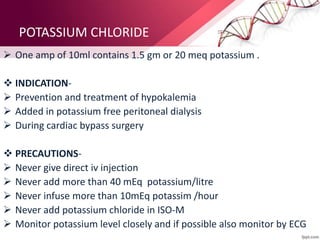
3. Ringer's lactate, isotonic saline, and 5% dextrose are preferred for diabetic patients as they do not contain glucose. NS, DNS, and dextrose solutions do not contain potassium and do not directly correct acid-

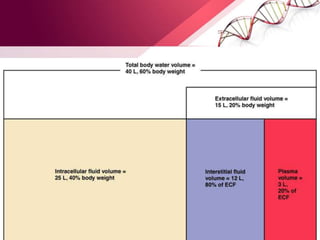
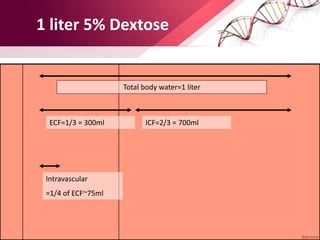
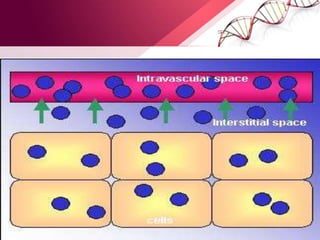
![Body Water Compartments
• Intracellular water: 2/ 3 (40%)of TBW
• Extracellular water: 1/3 (20%)of TBW
- Extravascular water[interstitial]: 3/4 (15%)of extracellular water
- Intravascular water[plasma]: 1/4 (5%)of extracellular water](https://image.slidesharecdn.com/principlesoffluidtherapy-170806183727/85/Principles-of-fluid-therapy-2-320.jpg)