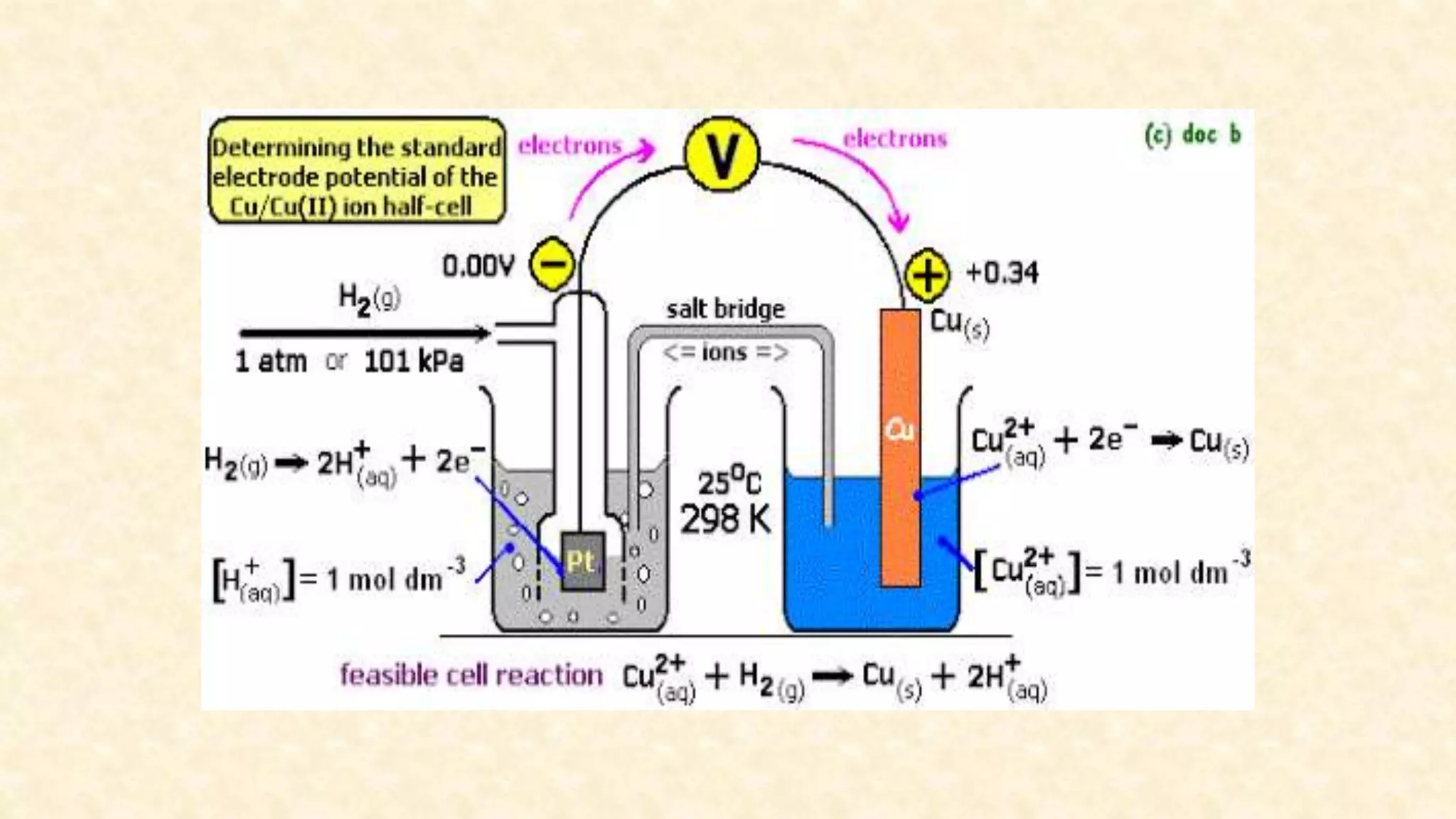
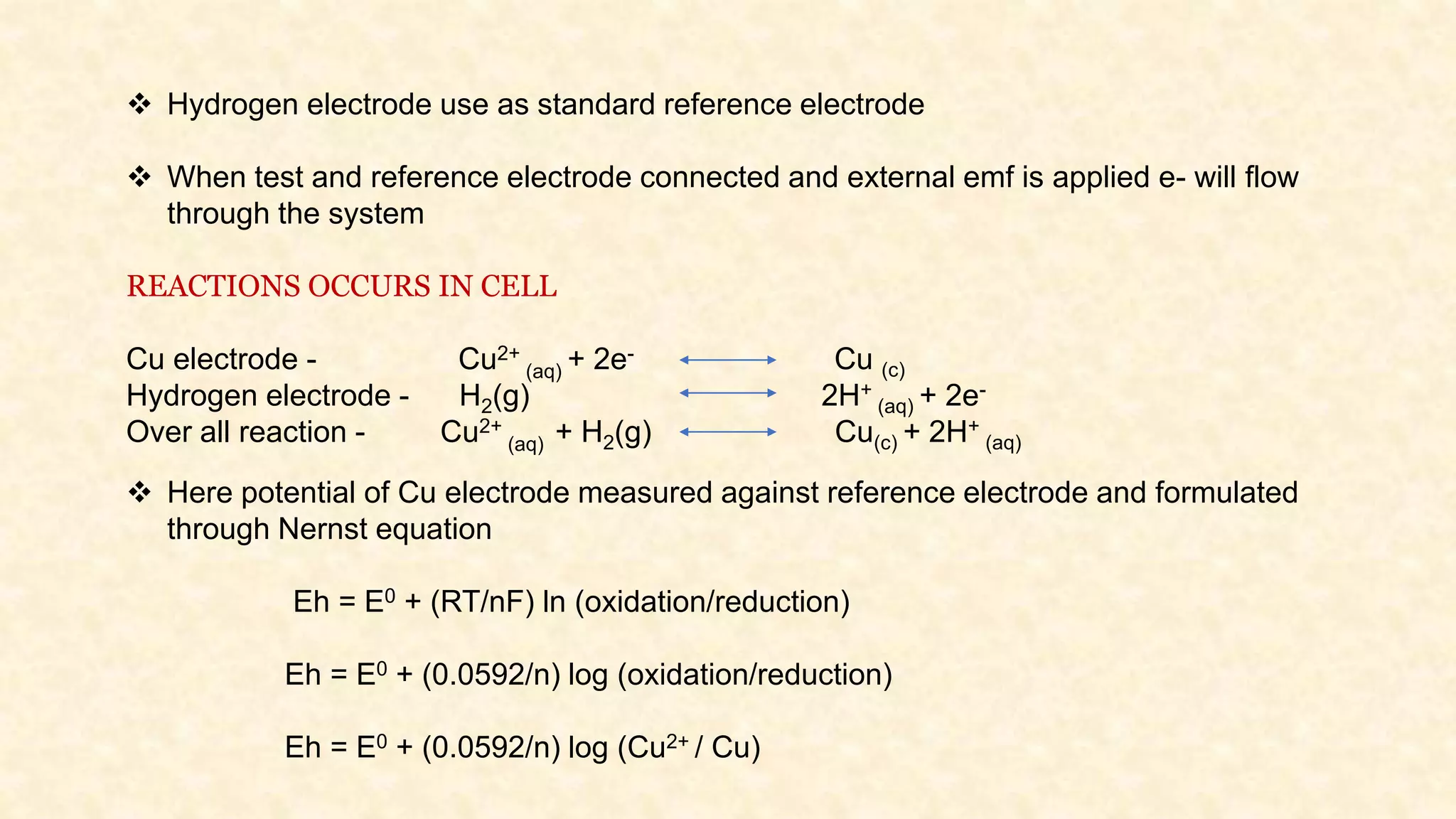
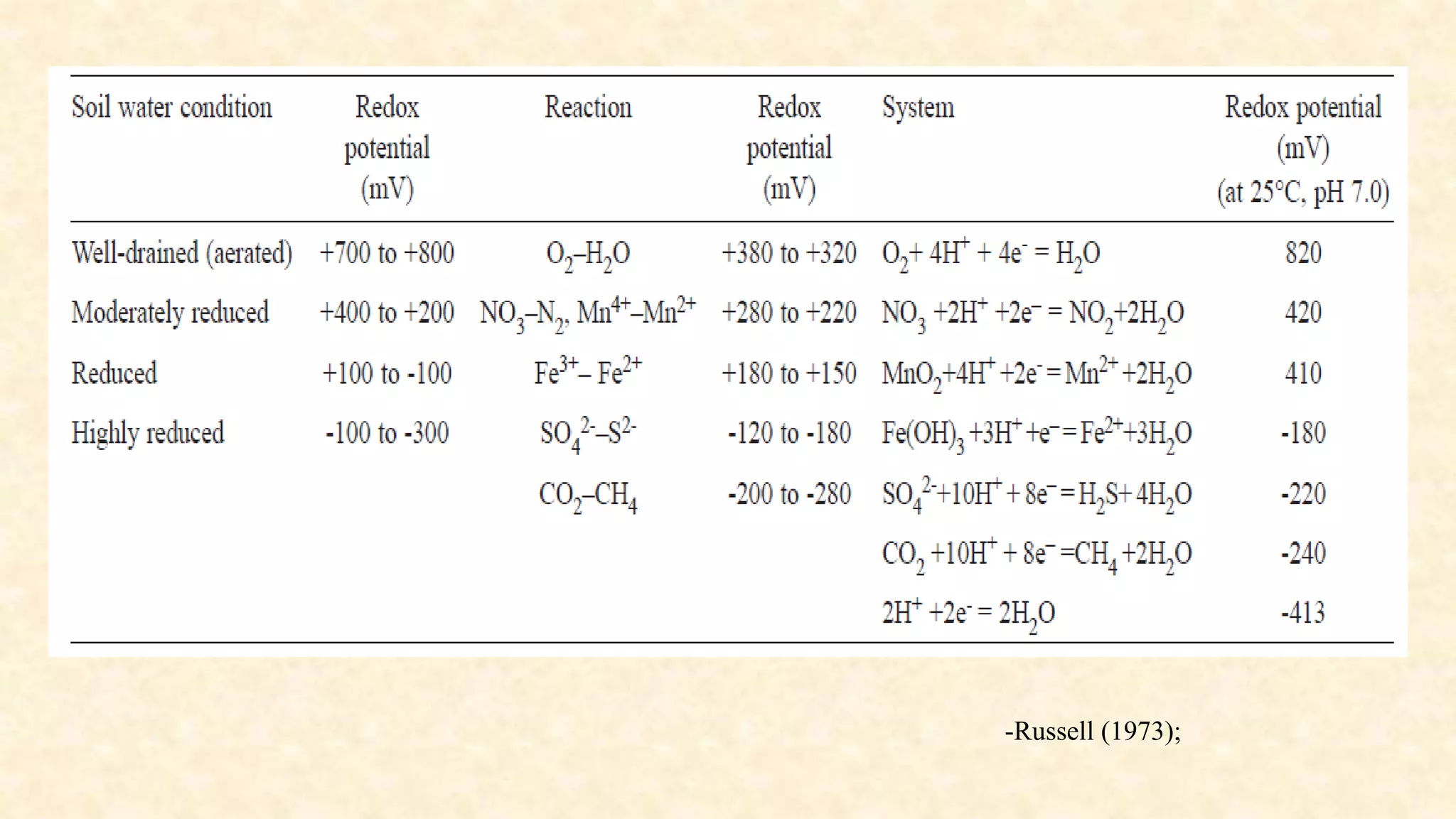
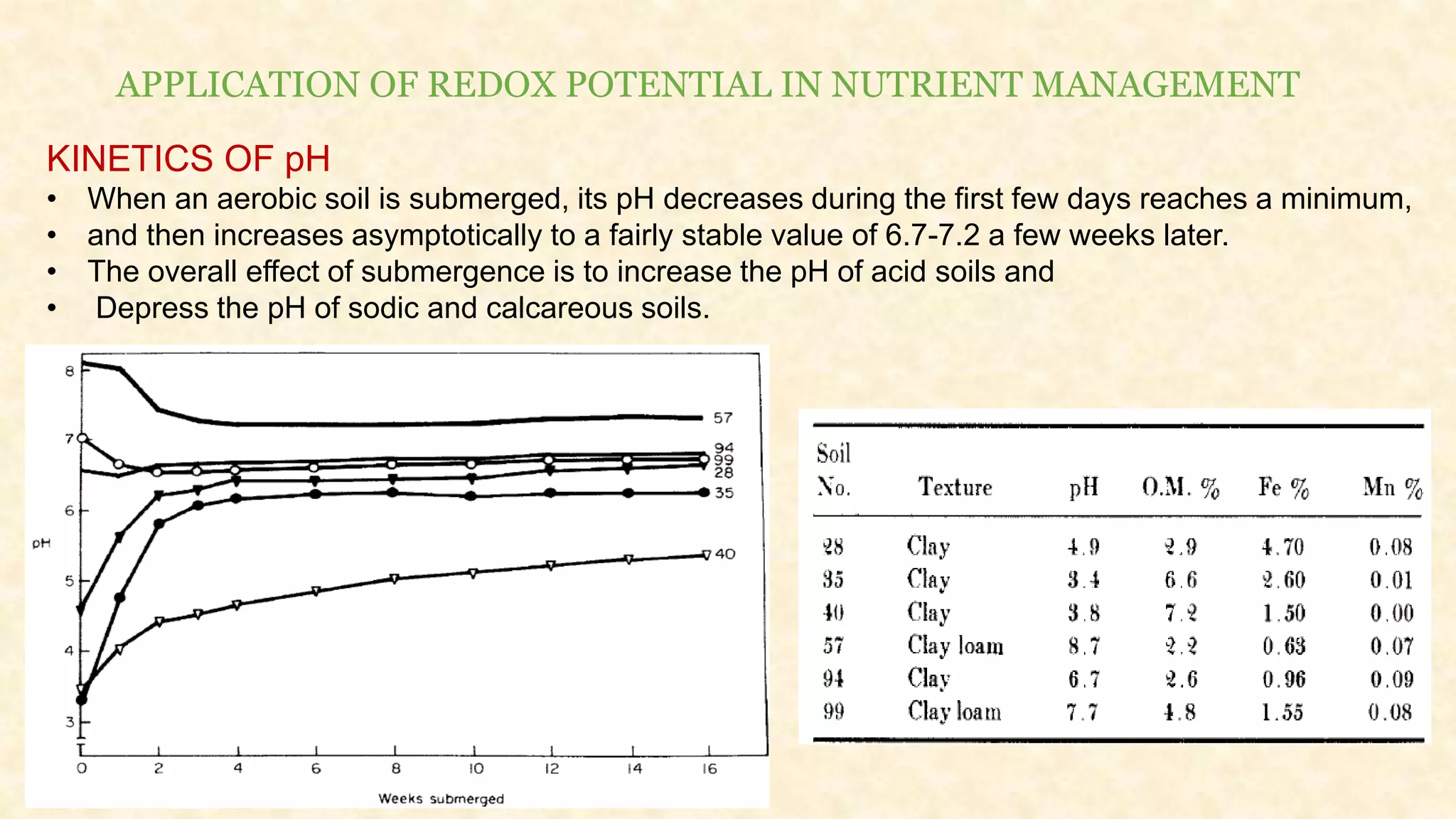
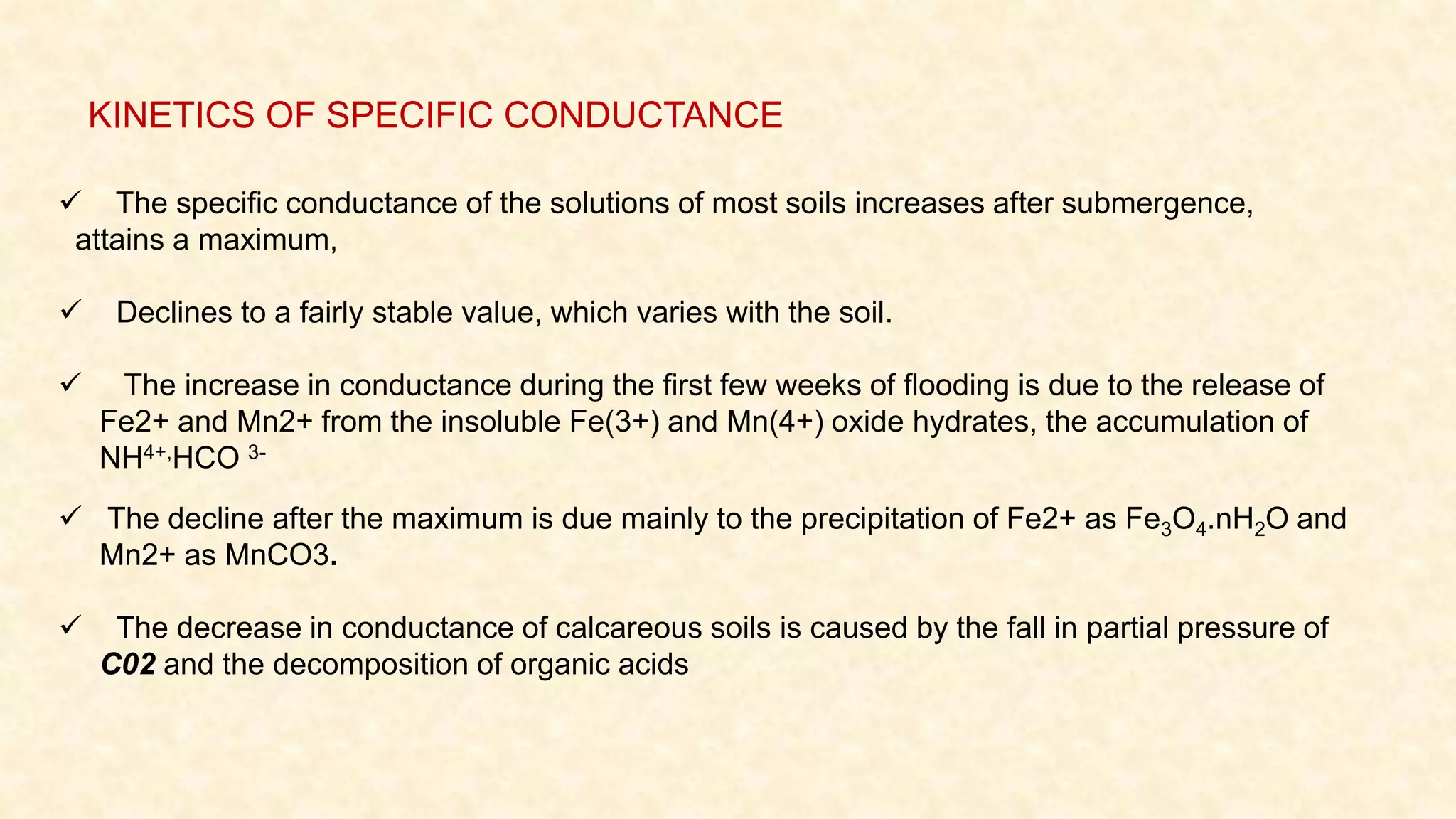
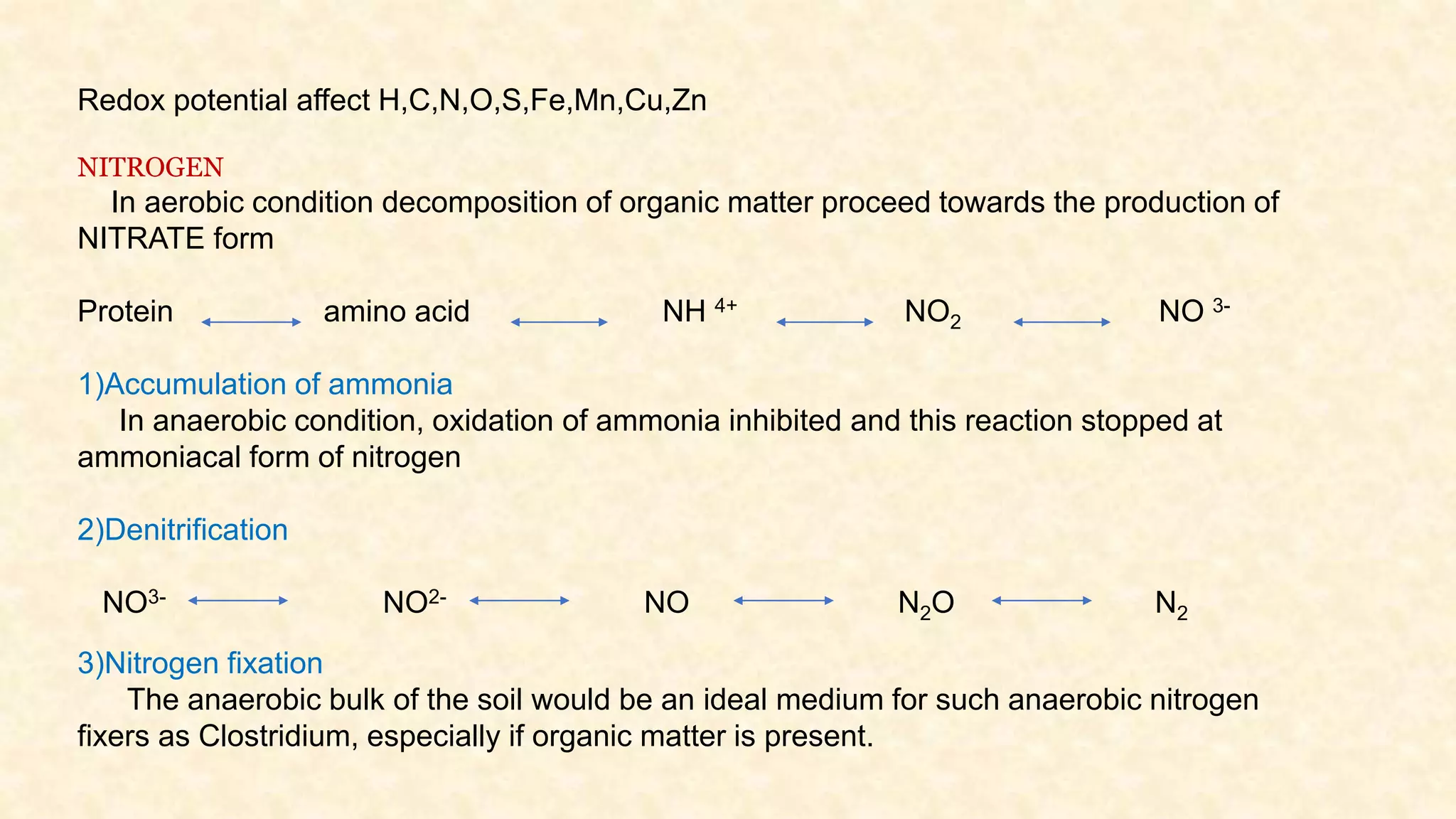
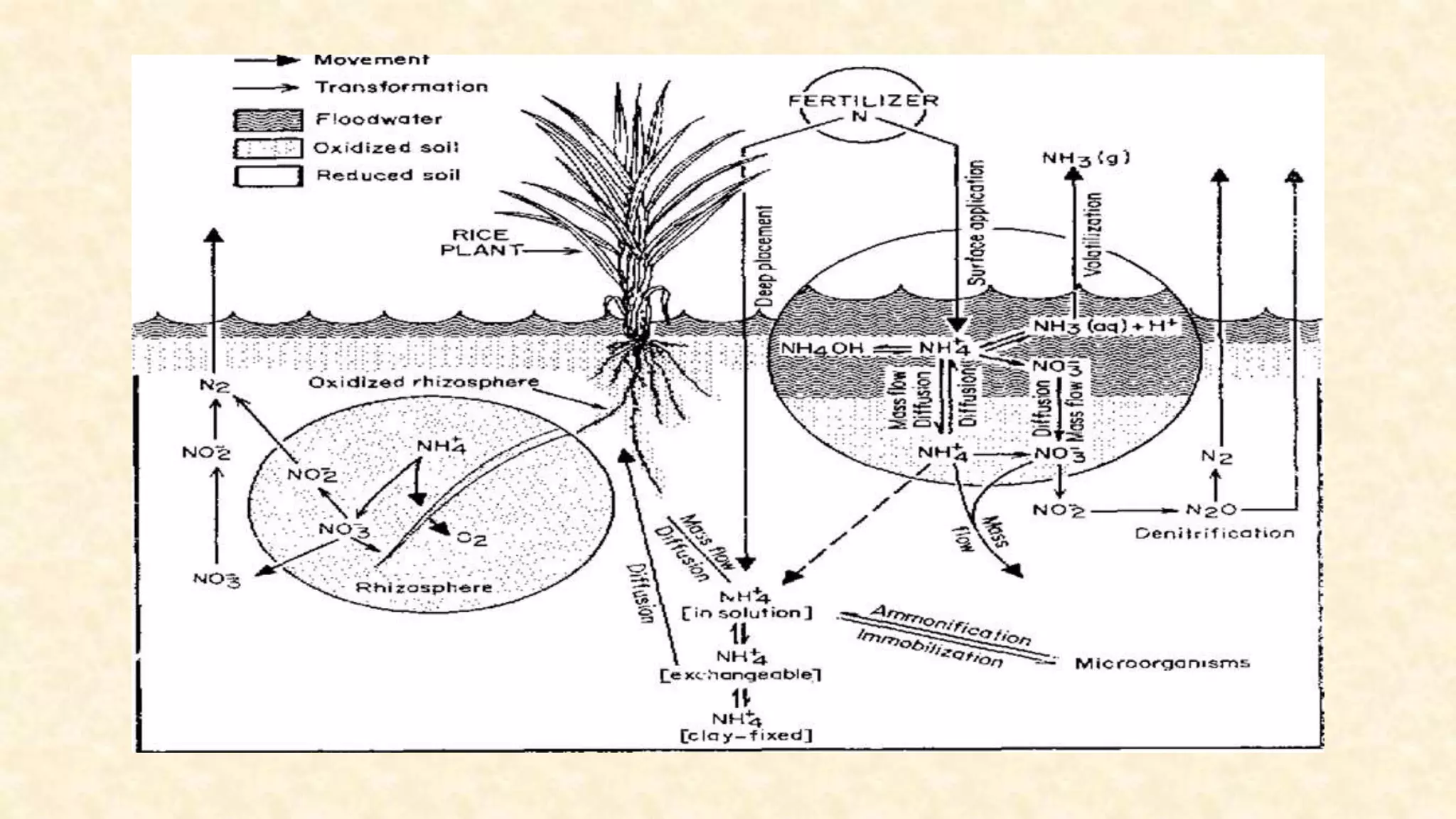
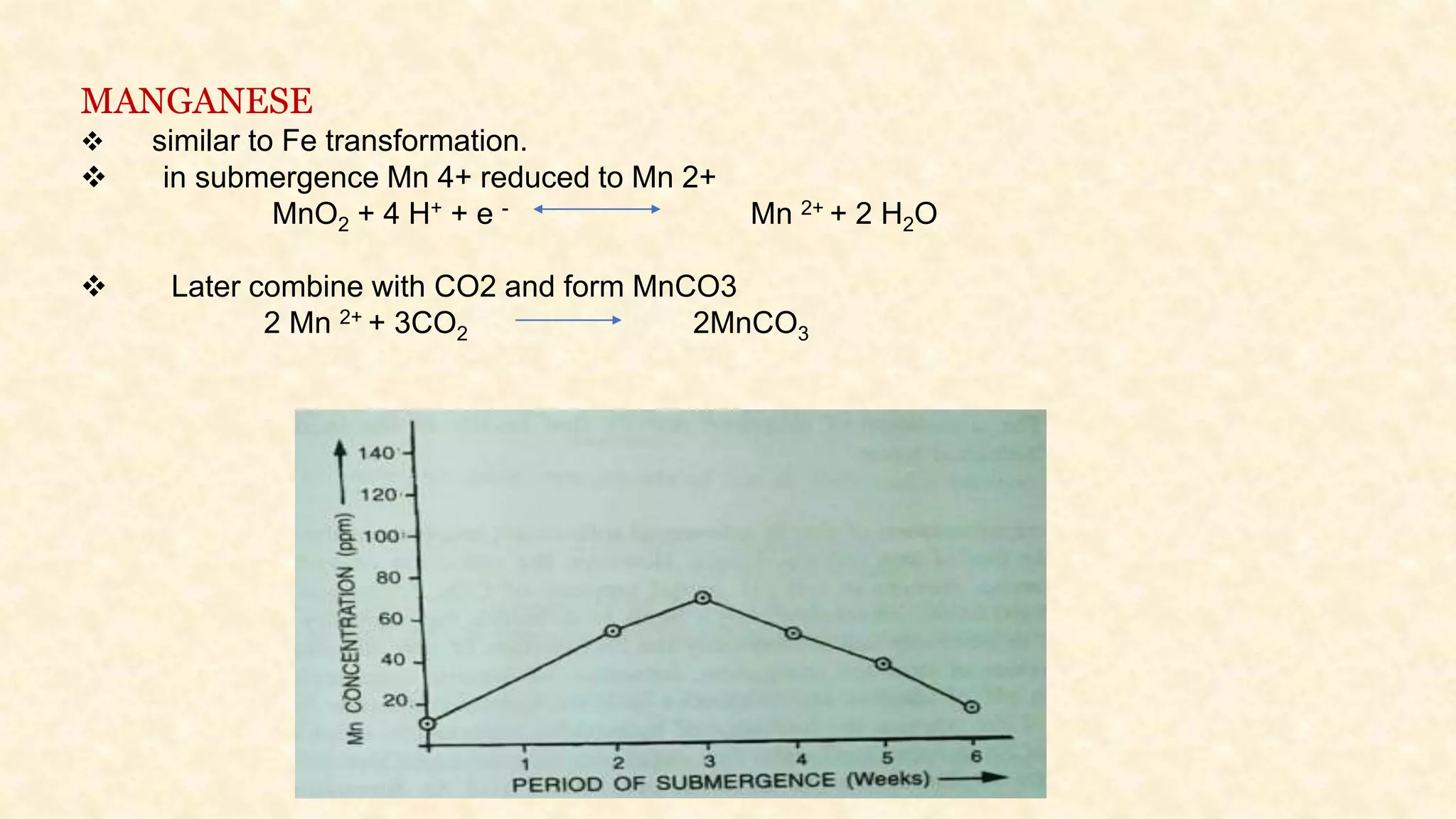
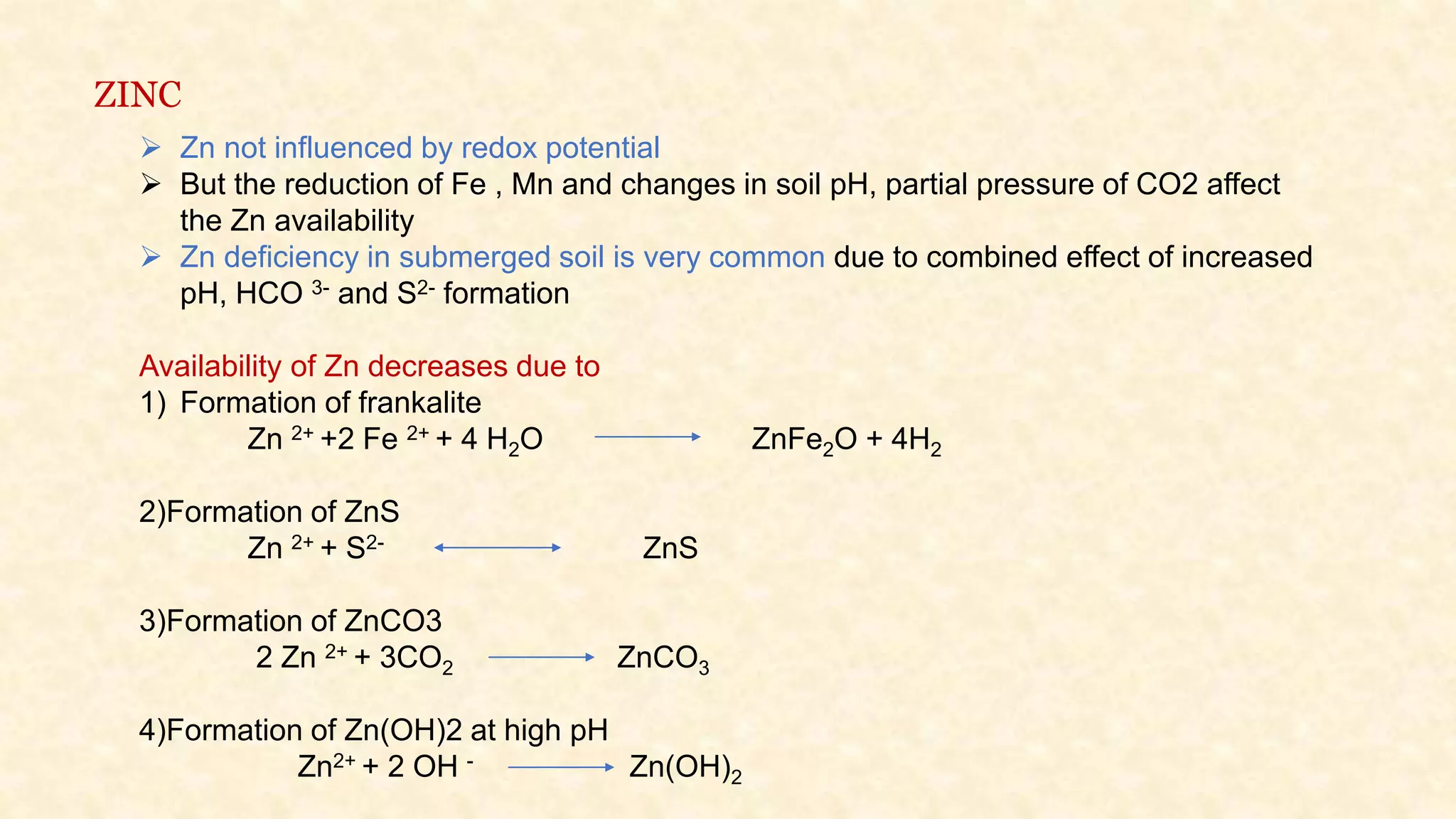
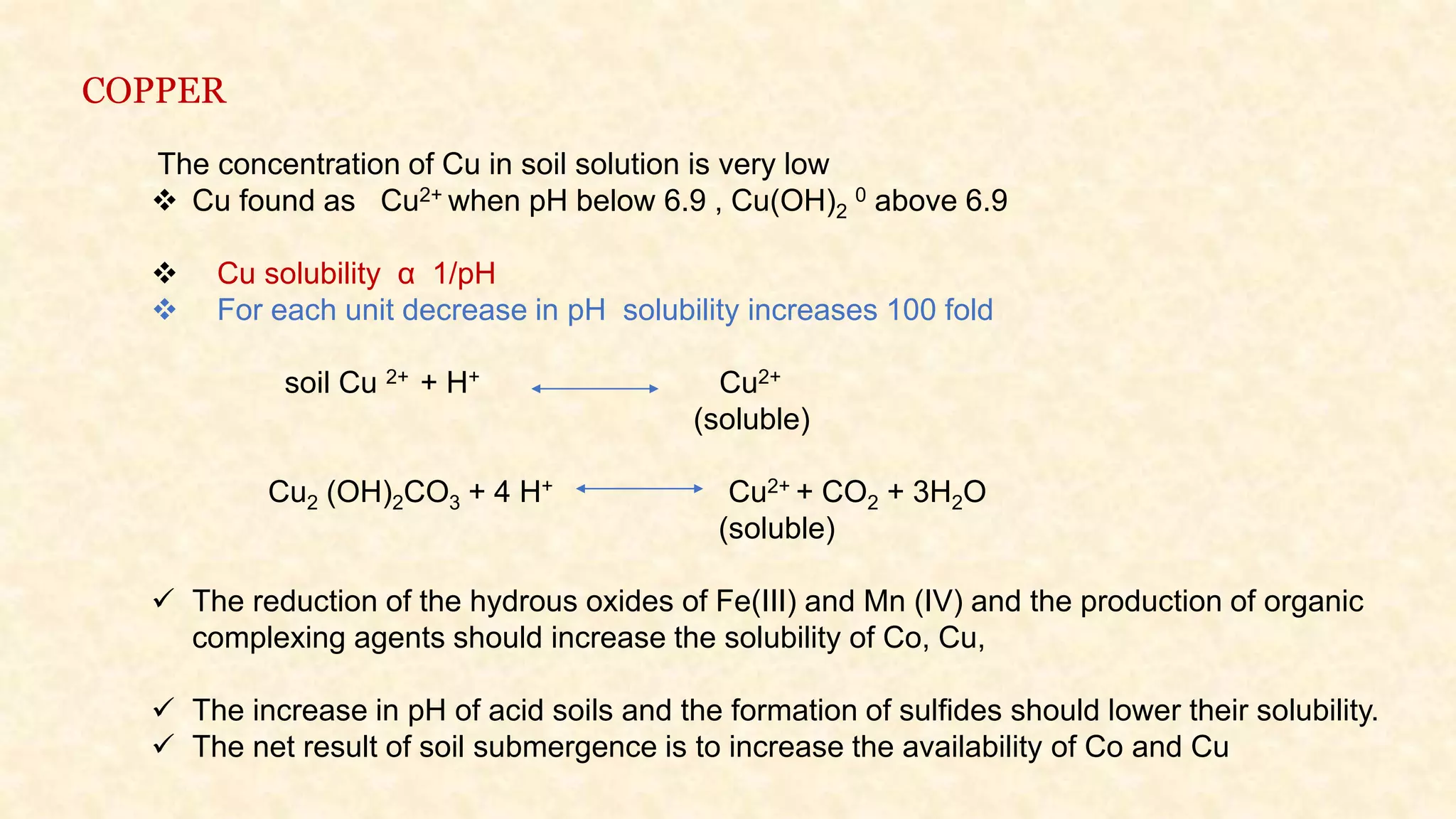
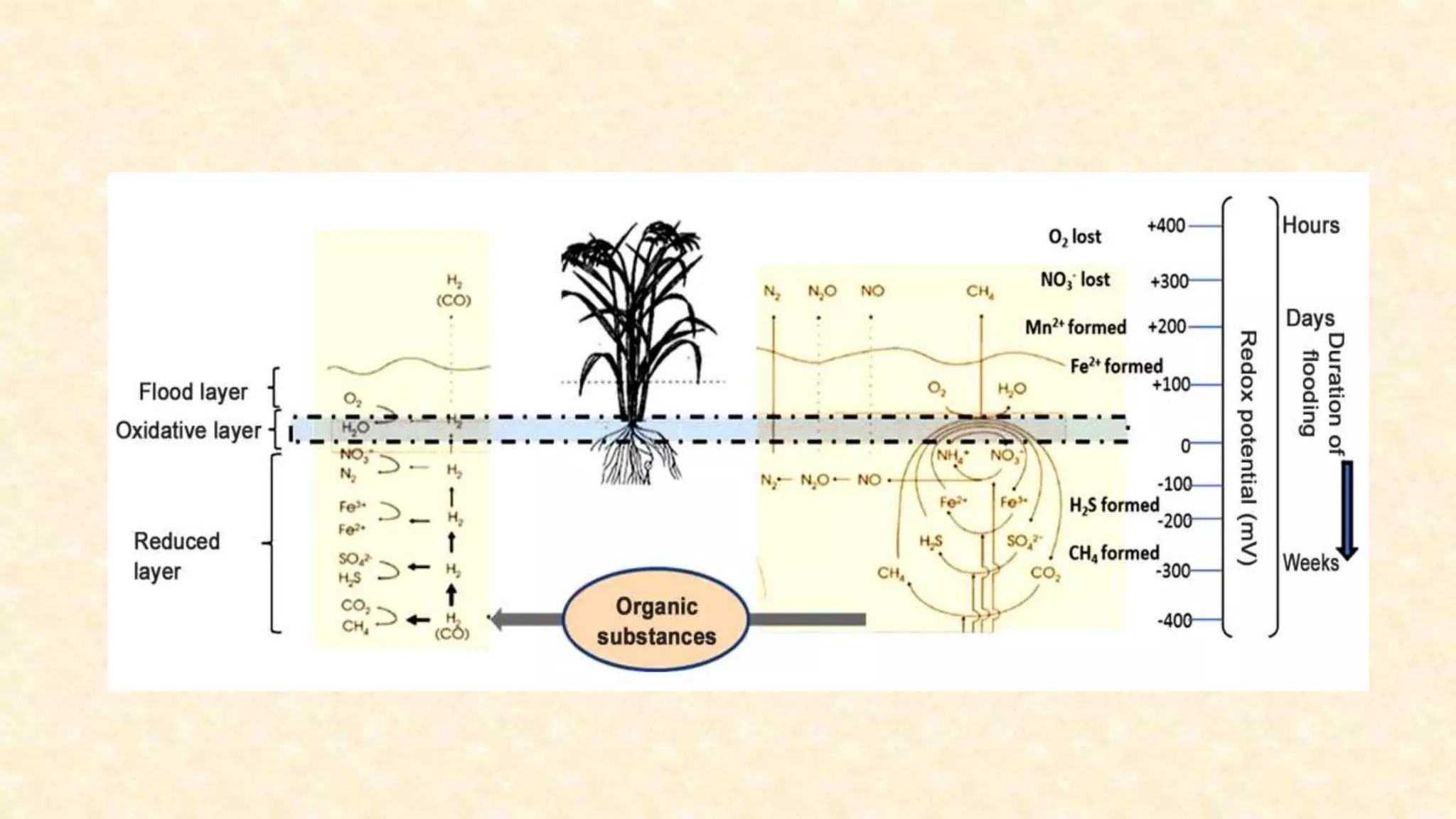
The document discusses the measurement of redox potential and its implications for soil fertility, focusing on soil chemistry and nutrient dynamics under submerged conditions. It explains how redox reactions and the resulting changes in electron activity affect various soil nutrients, including nitrogen, phosphorus, potassium, sulfur, iron, manganese, zinc, and copper. Additionally, the document presents analytical techniques for measuring redox potential and highlights the significance of these measurements in understanding soil fertility and nutrient availability.