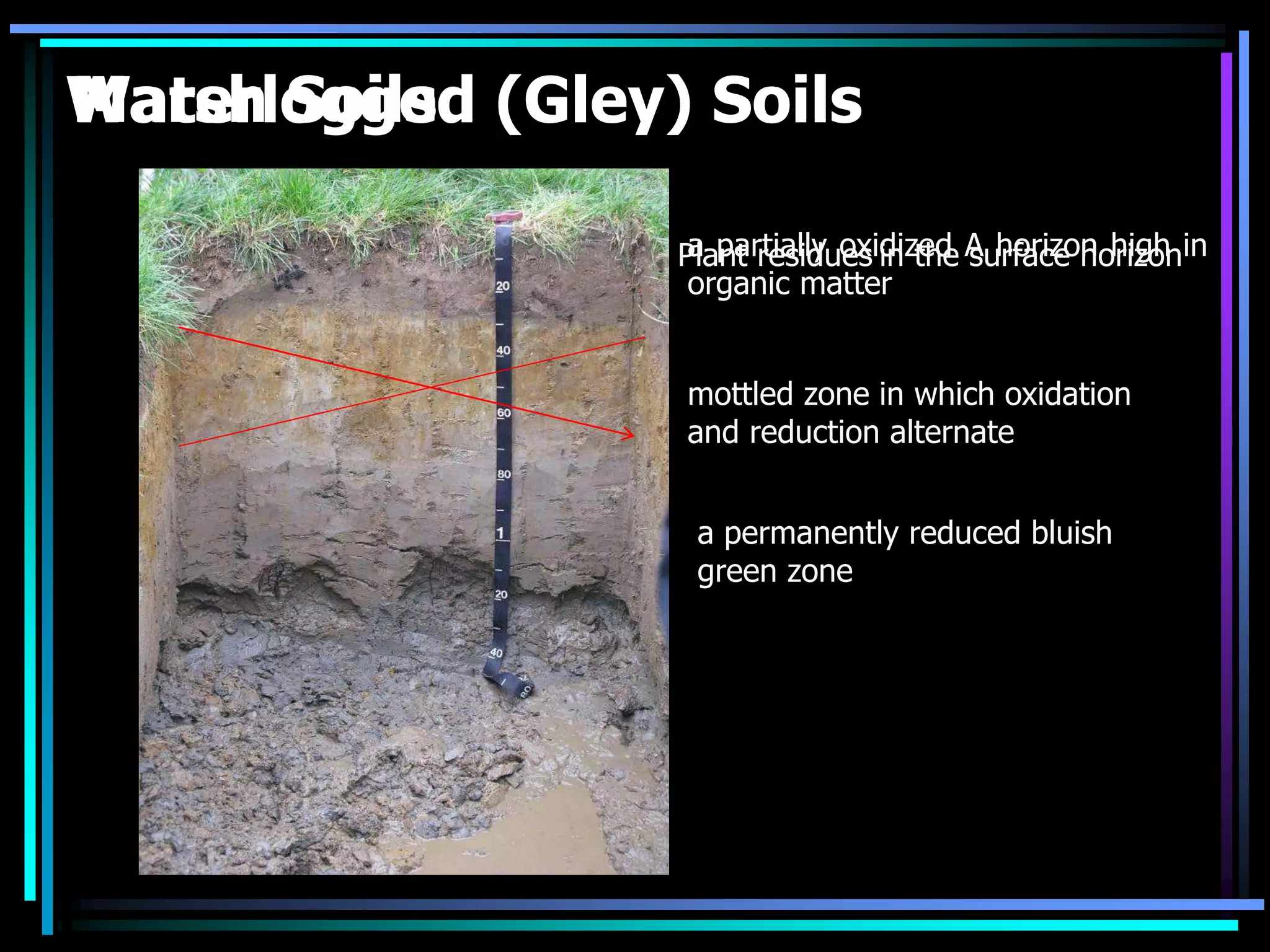
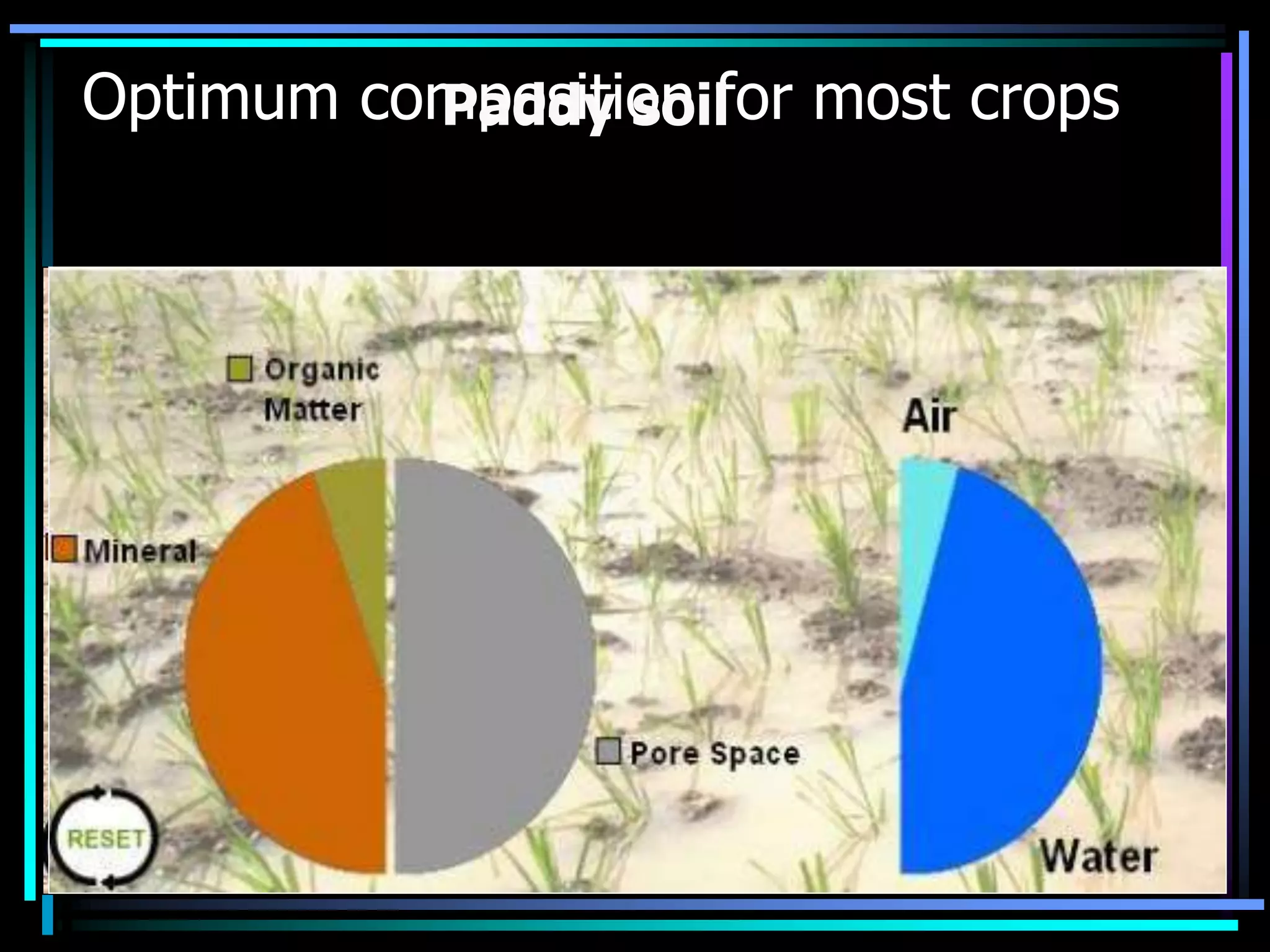
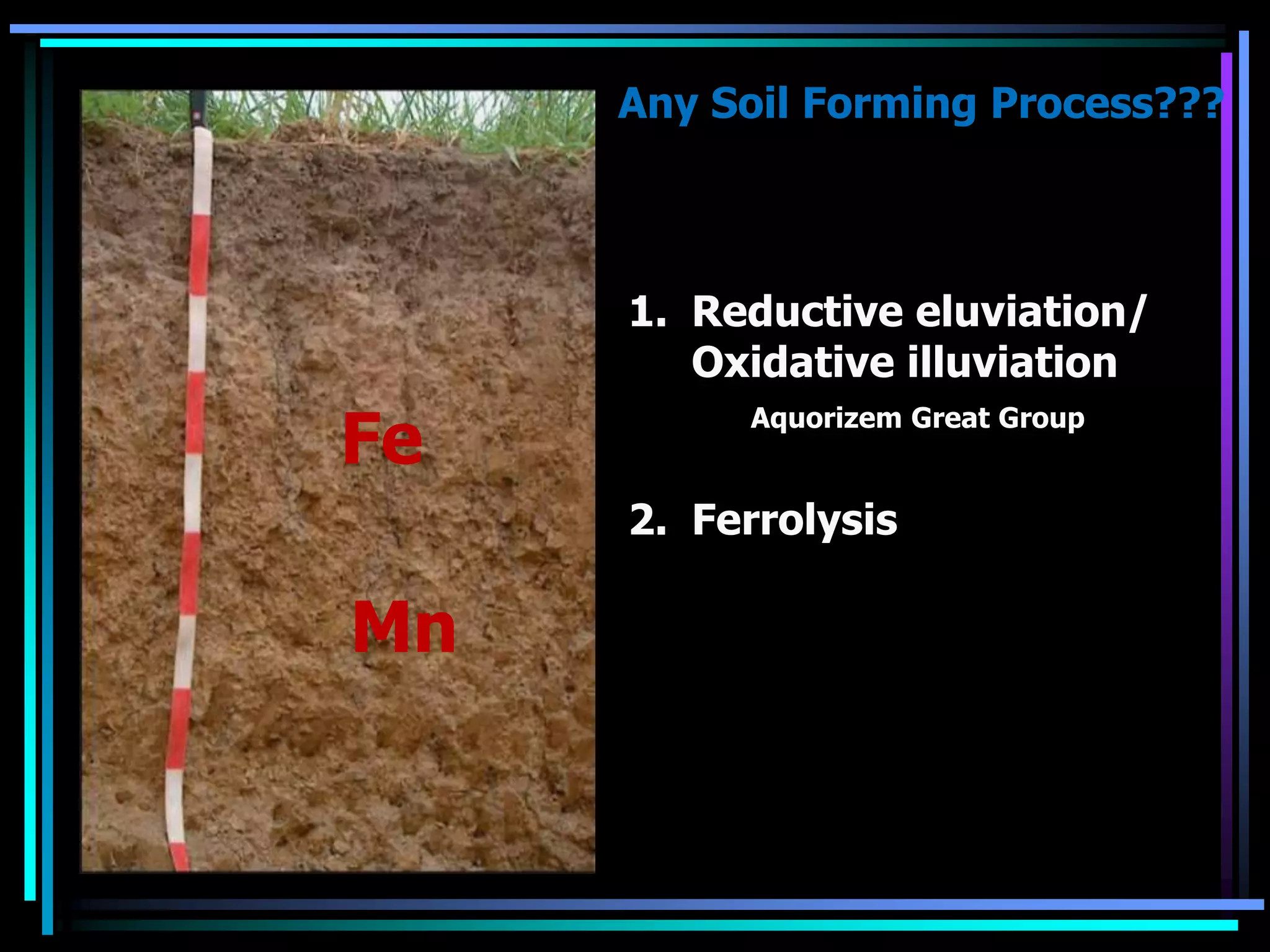
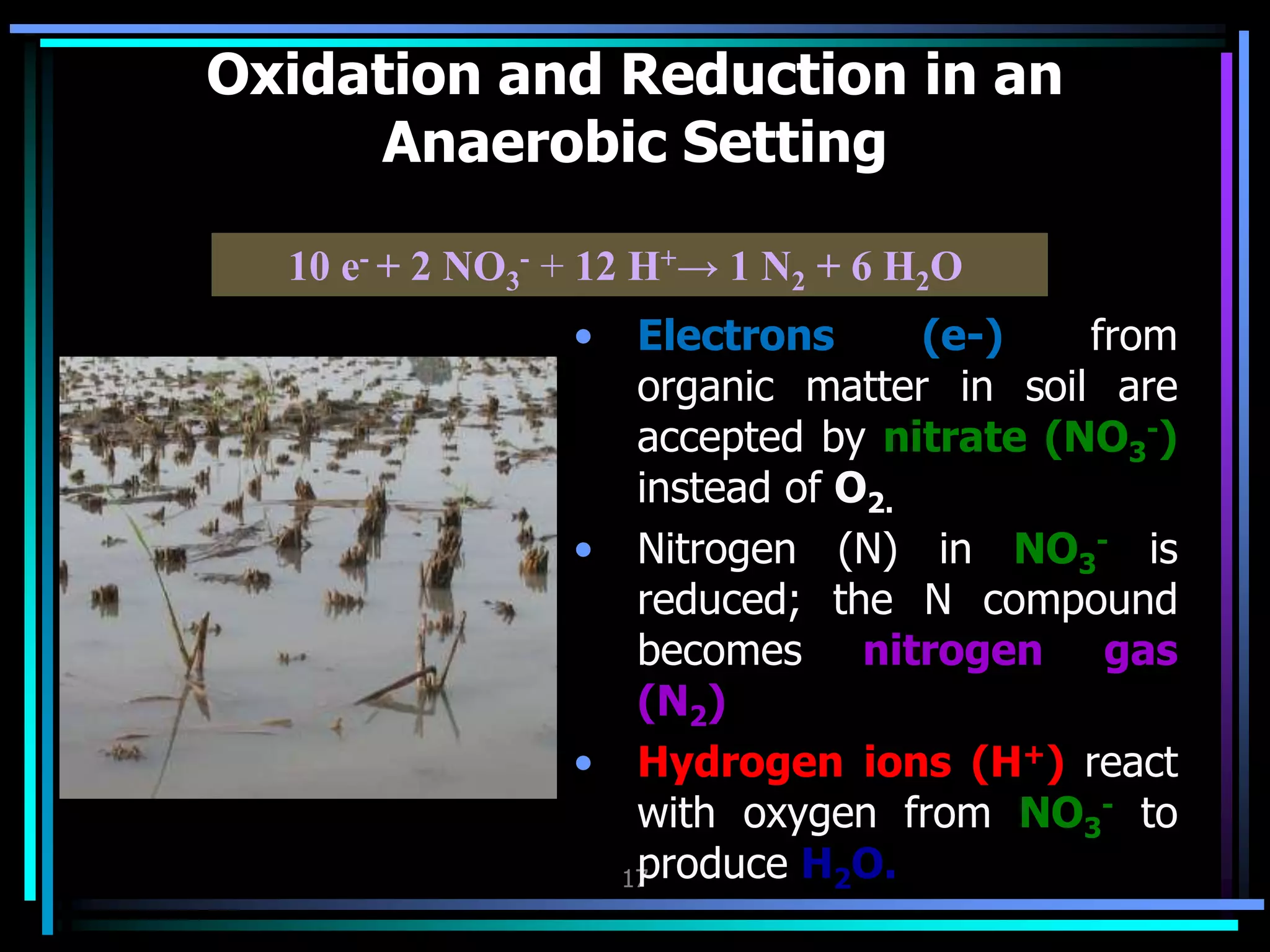
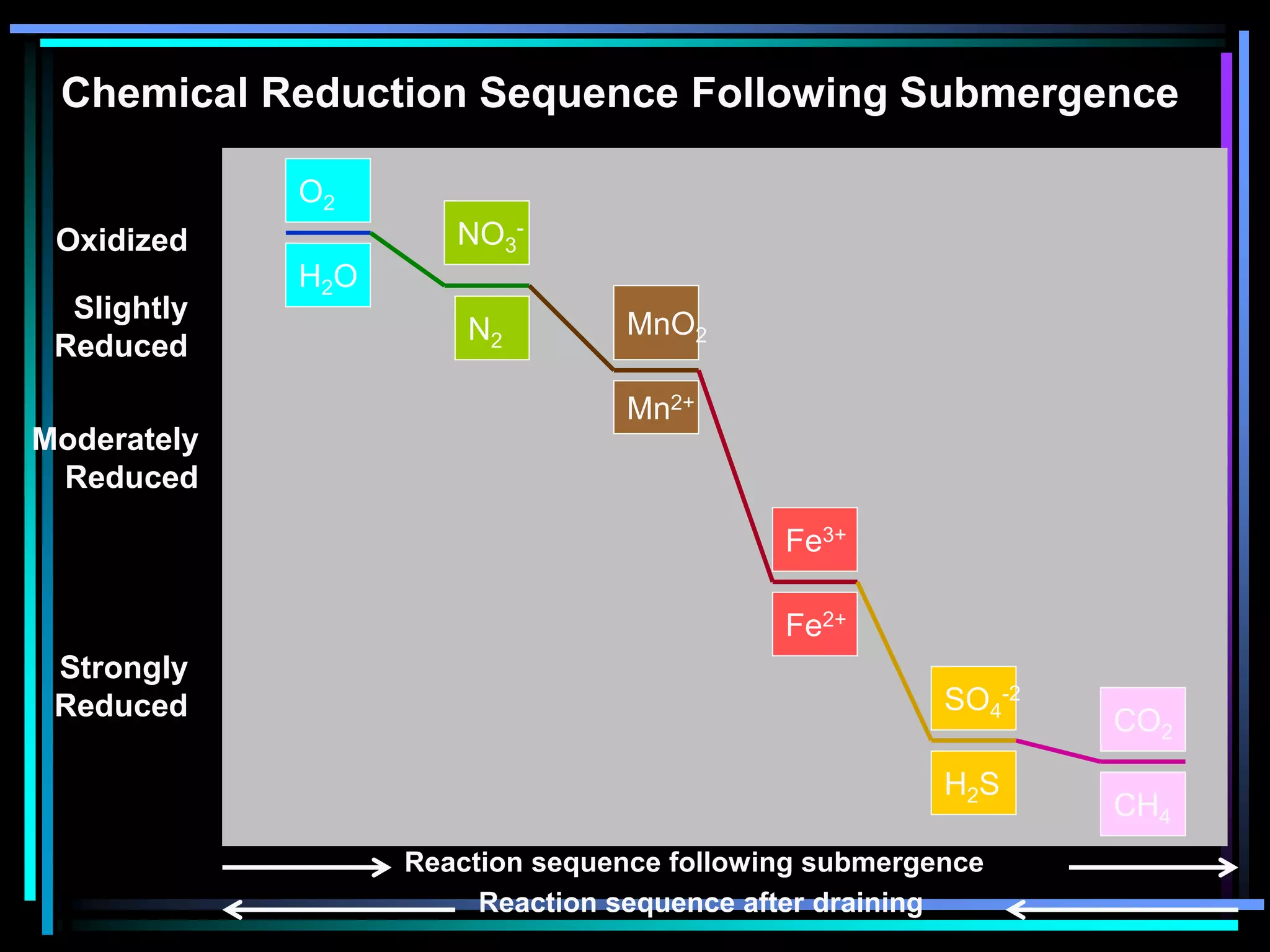
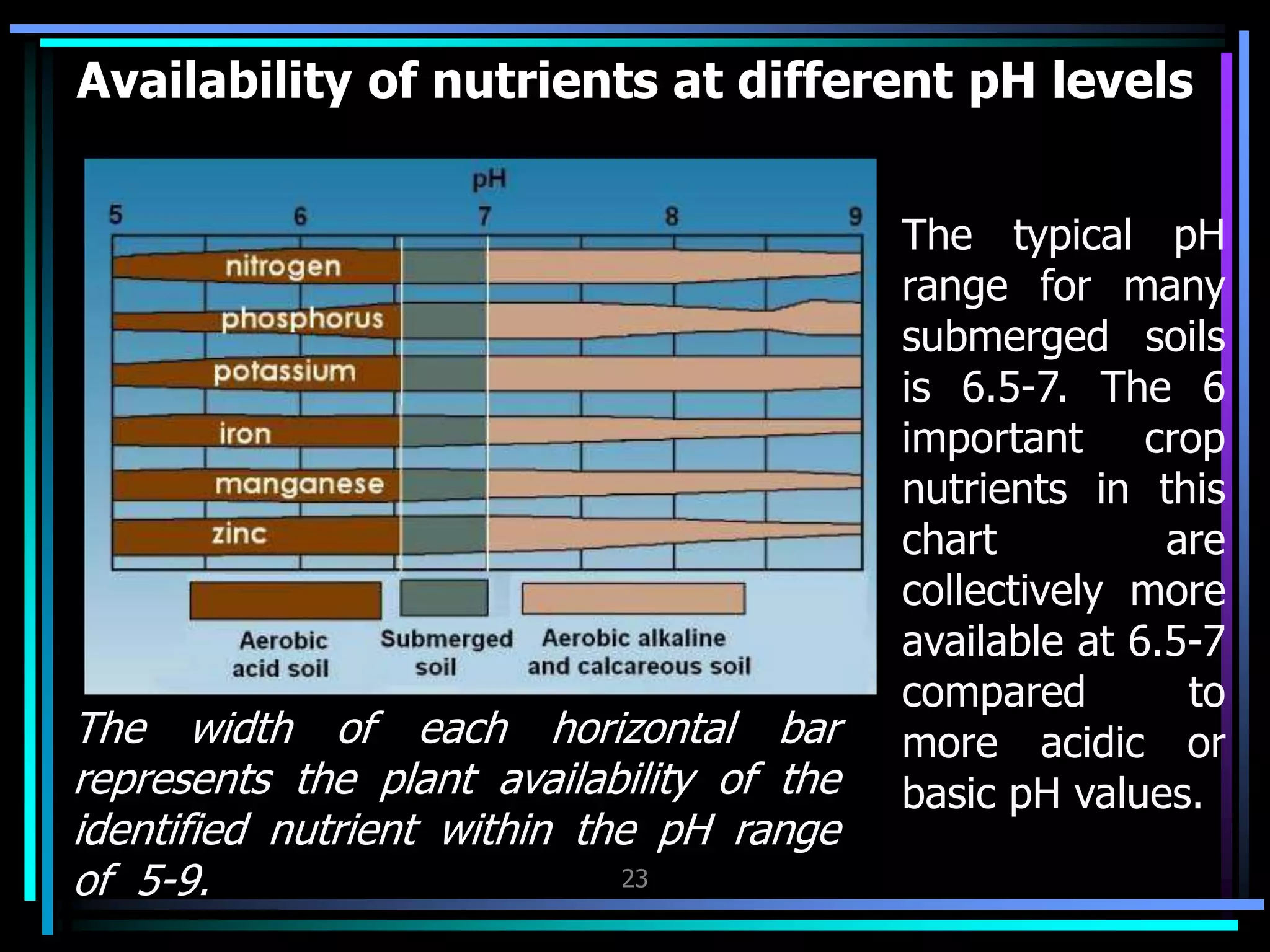
This document discusses soil chemistry in submerged soils. It explains that submergence leads to a lack of oxygen in the soil, causing a shift from aerobic to anaerobic organisms. Anaerobic respiration causes chemical compounds other than oxygen to be reduced in a predictable sequence from the least to most energetically favorable. This results in changes to the chemical forms of elements like nitrogen, iron, and sulfur in the soil. Submergence also typically causes soil pH to become more neutral. Nutrient availability is highest within this neutral pH range common for submerged soils.