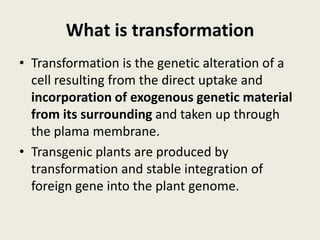
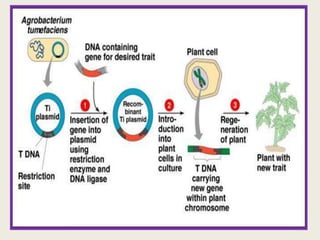
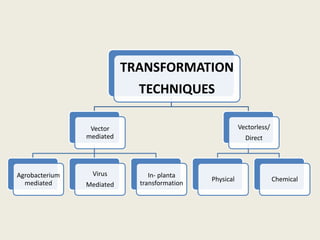
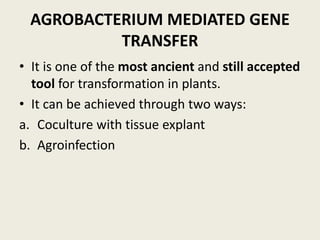
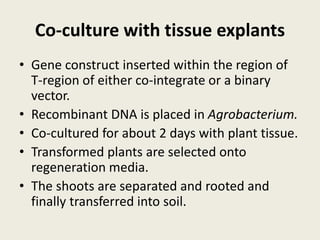
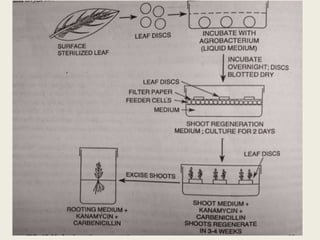
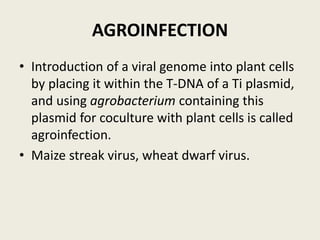
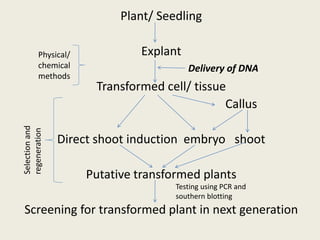
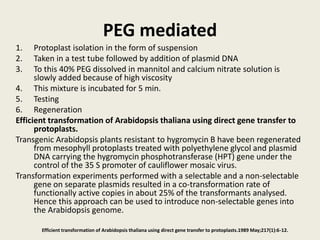
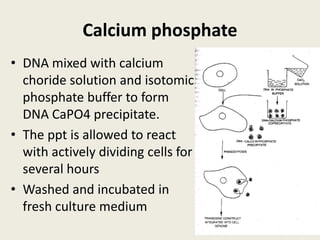
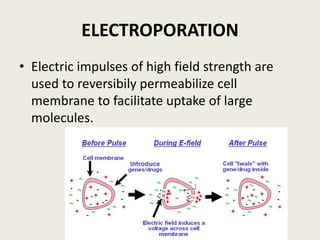
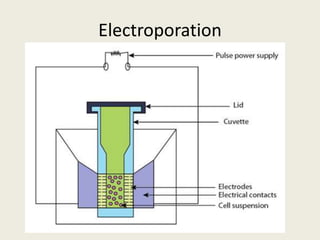
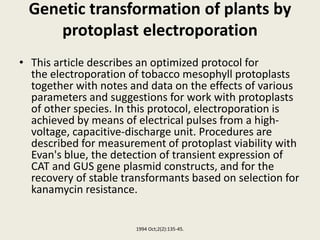
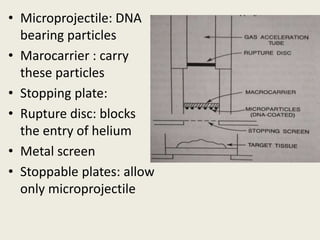
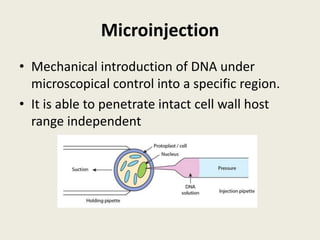
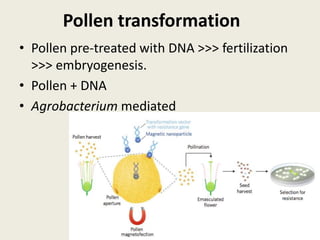
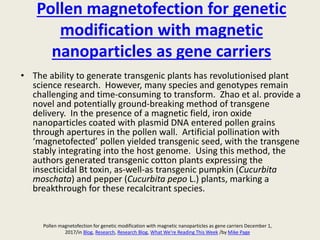
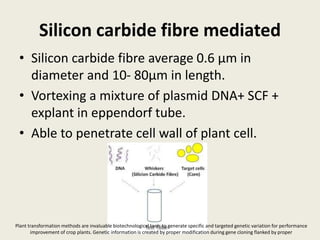
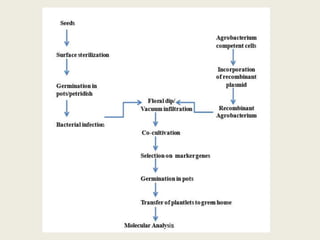
The document discusses the recent developments in plant transformation strategies, focusing on various transformation methods including Agrobacterium-mediated, virus-mediated, and direct physical/chemical techniques. It outlines the historical milestones in plant transformation, the mechanisms involved, and the advantages of in-planta transformation methods over traditional tissue culture methods. Additionally, it highlights novel approaches such as pollen magnetofection for genetic modification, aiming to enhance the efficiency and effectiveness of transforming challenging plant species.