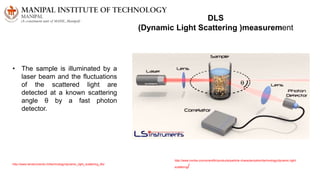
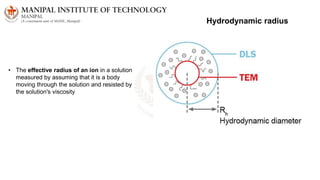
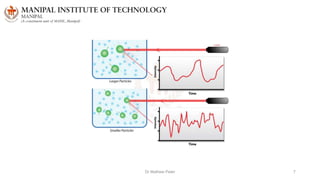
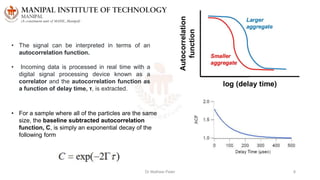
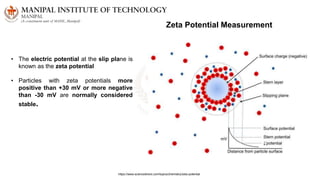
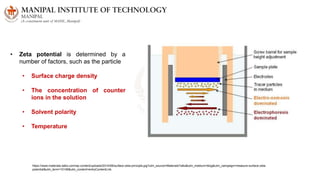
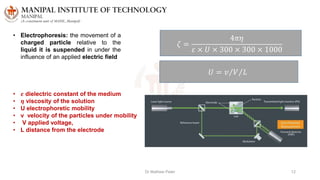
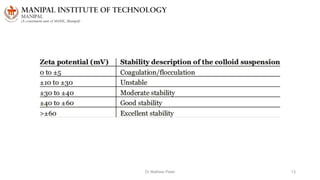
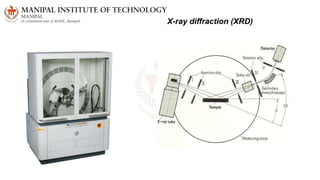
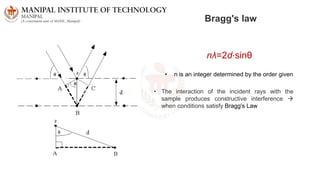
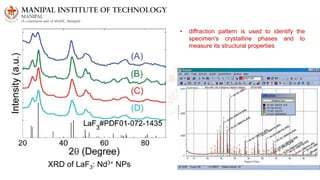
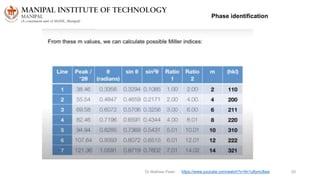
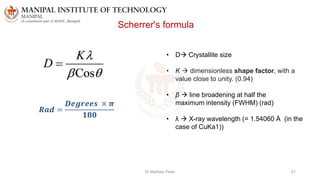
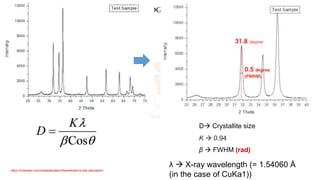
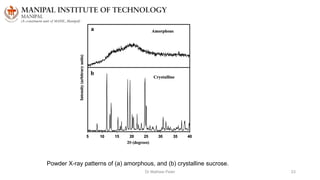
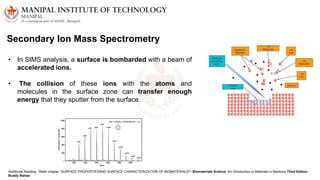
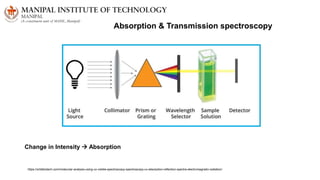
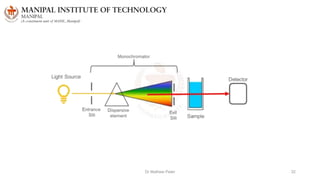
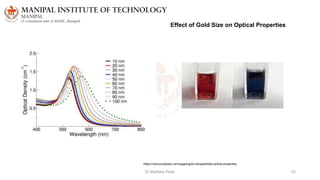
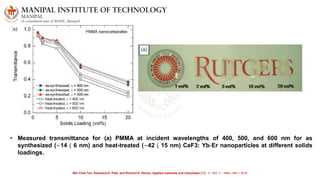
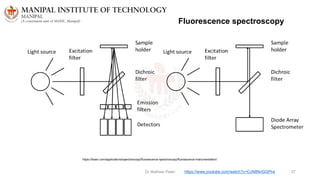
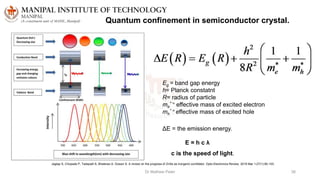
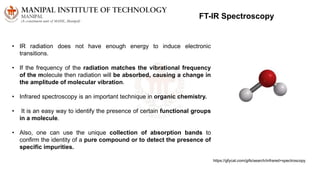
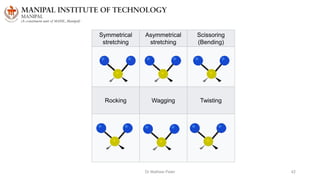
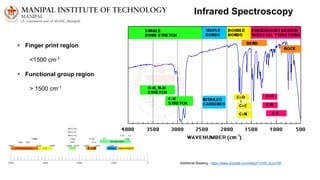
The document discusses various characterization techniques for nanomaterials, including dynamic light scattering (DLS), zeta potential measurement, X-ray diffraction (XRD), electron spectroscopy for chemical analysis (ESCA)/X-ray photoelectron spectroscopy (XPS), secondary ion mass spectrometry (SIMS), ultraviolet-visible spectroscopy (UV-Vis), fluorescence spectroscopy, Fourier transform infrared spectroscopy (FTIR), and their principles and applications. DLS measures hydrodynamic radius of particles in solution. Zeta potential indicates particle stability. XRD and XPS provide information on crystal structure, composition and bonding. SIMS and FTIR allow analyzing surface composition and bonds. UV-Vis, fluorescence and FTIR give information on optical and