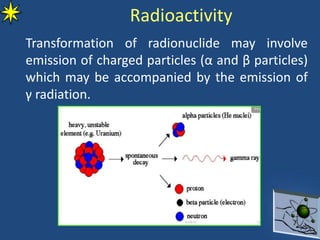
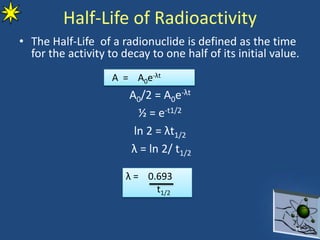
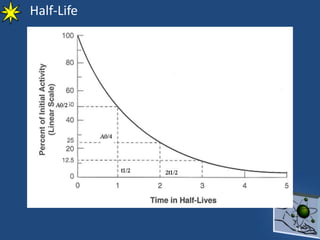
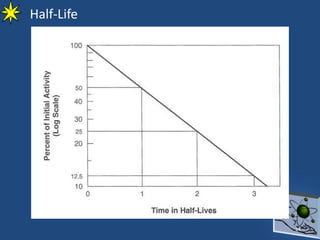
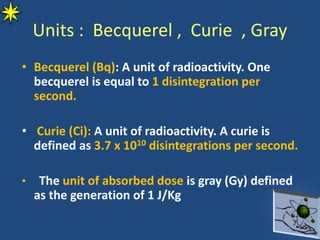
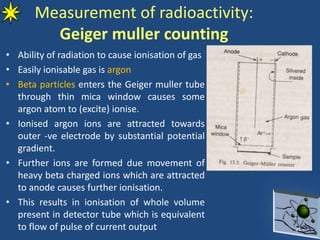
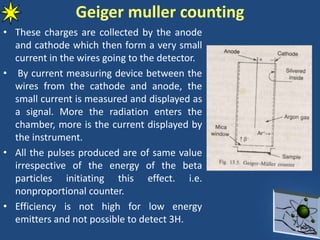
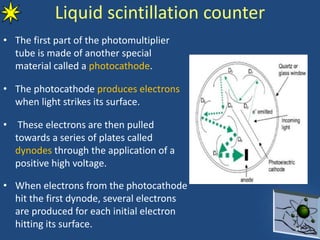
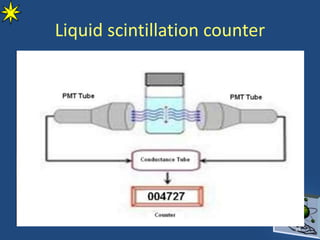
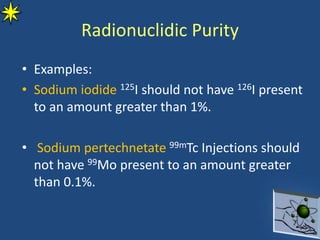
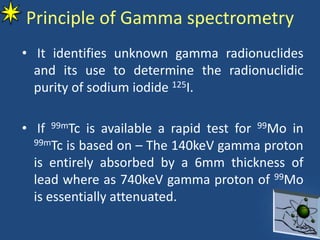
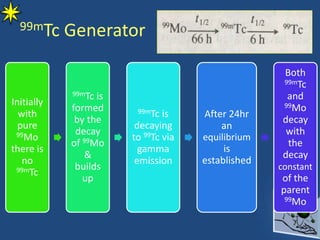
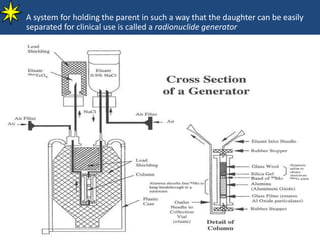
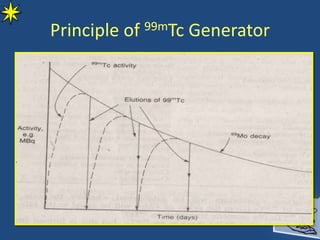
This document provides information on radiochemistry and radiopharmaceuticals. It discusses fundamentals of radioactivity including properties of radionuclides, radioactive decay, half-life, units of measurement. It also covers topics like radionuclidic purity, radiochemical purity, measurement techniques like Geiger-Muller counting and liquid scintillation counting. The document discusses quality control of radiopharmaceuticals and safety aspects of working in a radiopharmaceutical laboratory.