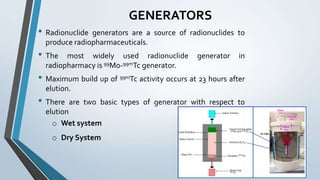
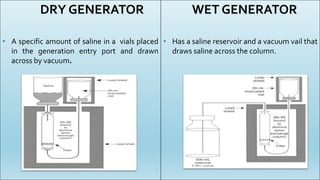
This document summarizes key aspects of radiopharmaceuticals used in nuclear medicine. It describes what radiopharmaceuticals are and their main components. It also discusses common equipment used like generators, dose calibrators and contamination monitors. Different types of cold kits are presented for preparing radiotracers like Tc-99m MDP for bone scans, Tc-99m DTPA for renal imaging, and Tc-99m sulfur colloid for liver scans. Ideal properties of radiopharmaceuticals are outlined as well as references for further reading.

















![KIT FOR [Tc]-BRIDA
BROMO-IMINODIACETIC ACID[Tc]-BRIDA
USE: STUDY OF LIVER &GALLBLADDER
PREPARTION:
• 20–30 mCi of TcO4 prepared in 1 ml of 0.9% saline
and introduced into the BRIDA kit
• Shaken and kept at room temperature for 30 min.
• The pH of the reconstituted product is 4.2 to 5.7
STORAGE : 2-80C](https://image.slidesharecdn.com/radiopharmaceuticalsalan-221228050516-c29d37fa/85/RADIOPHARMACEUTICALS-21-320.jpg)





![TYPE OF SCAN RADIOPHARMACEUTICAL
Bone Scan MDP
FOR RENAL AND BRAIN
SCINTIGRAPHY
DTPA
FOR RENAL CORTICAL SCANS DMSA
STUDY OF LIVER
&GALLBLADDER
[Tc]-BRIDA
LIVER SCAN SULPHUR COLLOID
THYROID SCAN TC 99M](https://image.slidesharecdn.com/radiopharmaceuticalsalan-221228050516-c29d37fa/85/RADIOPHARMACEUTICALS-27-320.jpg)

