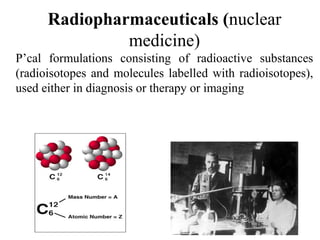
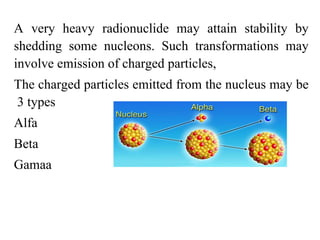
1) Radiopharmaceuticals are pharmaceutical formulations containing radioactive substances used for diagnosis and therapy. They emit radiation from radioactive decay which is detected by imaging devices.
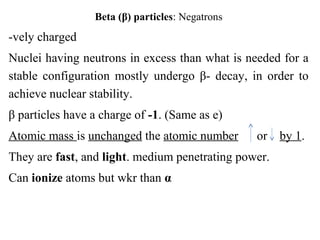
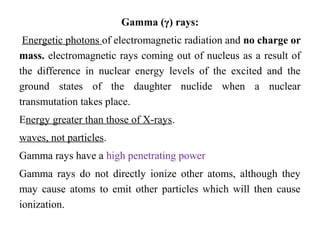
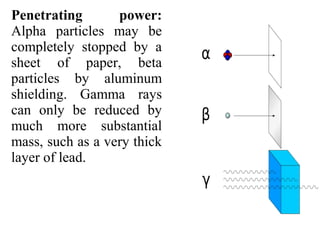
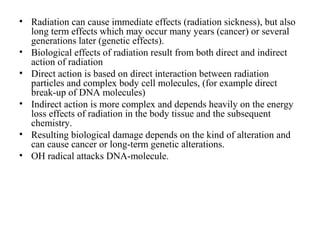
2) There are three main types of radiation emitted - alpha, beta, and gamma. Alpha particles have the lowest penetrating power while gamma rays have the highest.
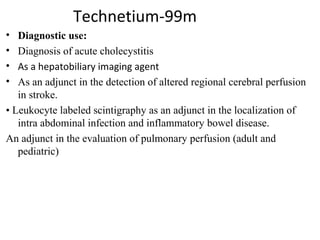
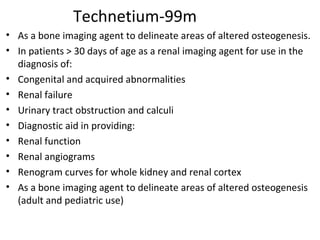
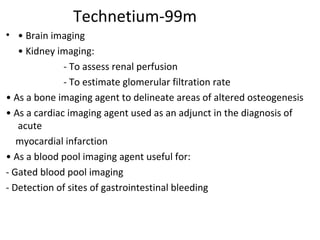
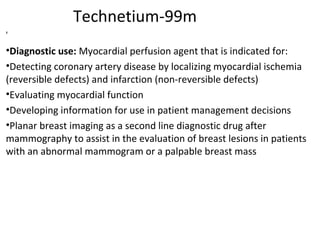
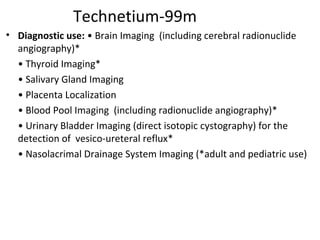
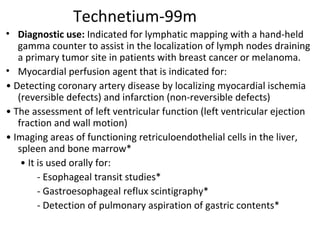
3) Common medical radiopharmaceuticals include technetium-99m, which is used for bone, kidney, brain and cardiac imaging, and iodine-125 which is used for thyroid imaging and function tests.