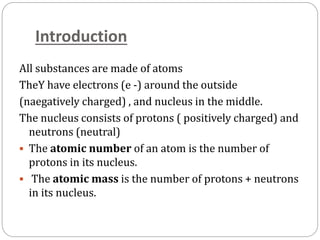
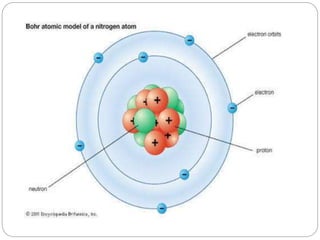
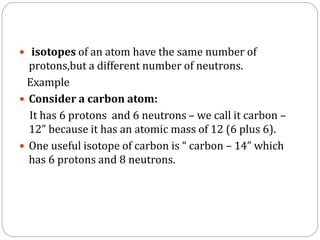
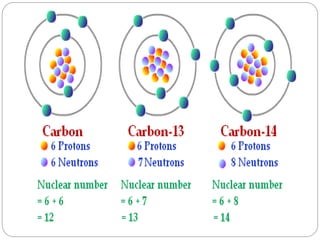
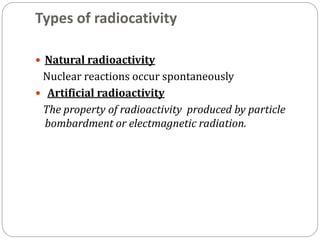
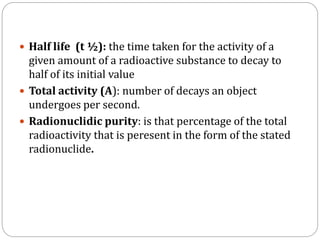
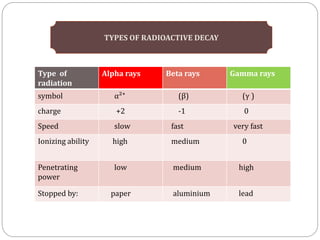
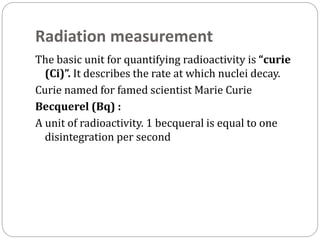
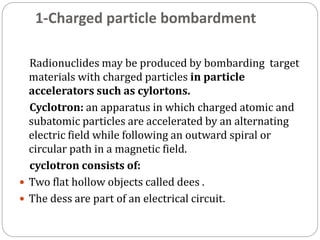
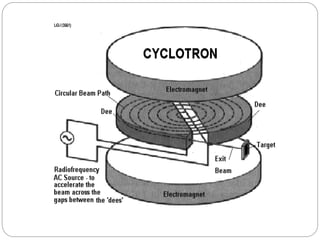
Radiopharmaceuticals are pharmaceutical drugs that contain radioactivity and can be used for diagnostic or therapeutic purposes. They are produced via several methods including charged particle bombardment, neutron bombardment, or using a radionuclide generator. Proper preparation of radiopharmaceuticals involves compounding, sterilization, and quality control checks for visual inspection, radioactivity levels, radionuclidic purity, radiochemical purity, microbiological sterility, and labeling. Radiopharmaceuticals must be properly shielded and have a short shelf life due to radioactive decay.