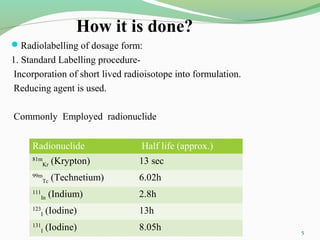
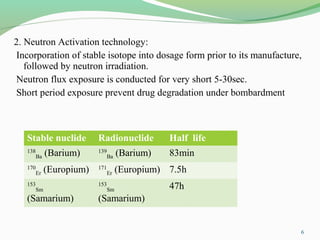
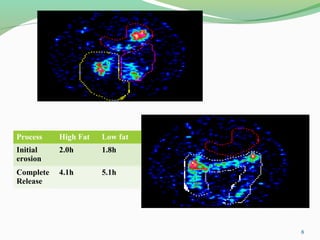
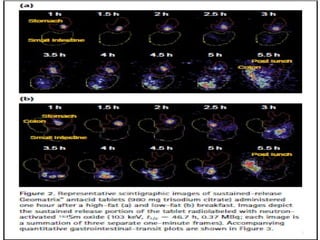
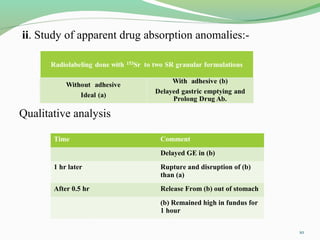
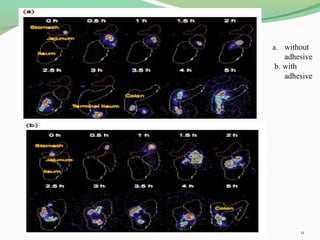
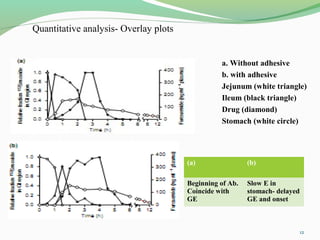
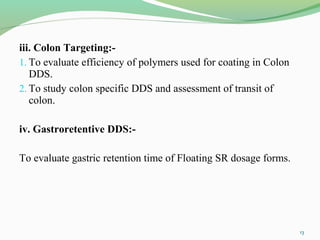
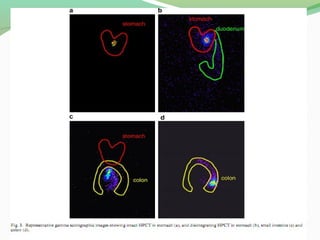
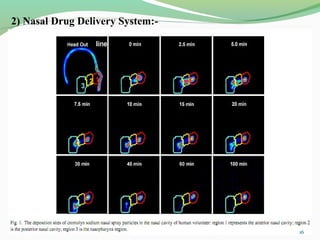
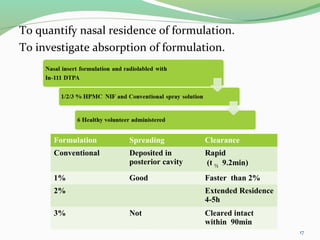
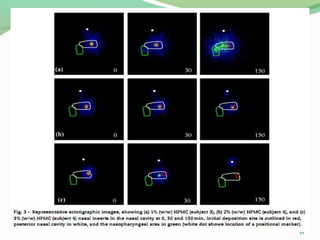
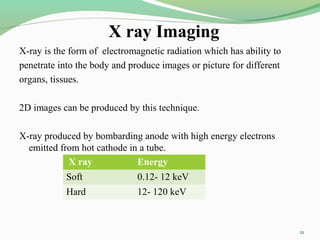
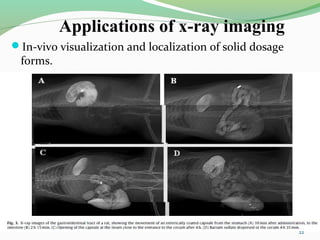
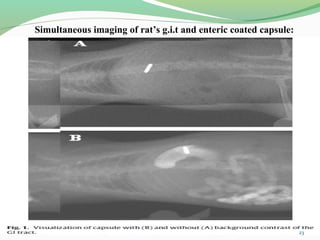
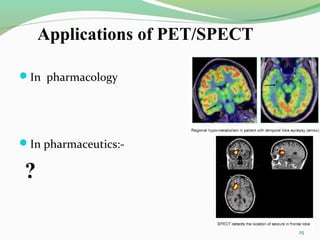
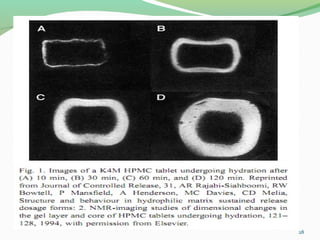
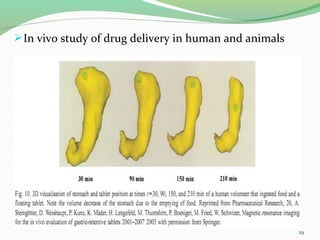
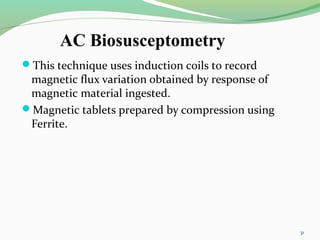
The document discusses various radio imaging techniques, primarily focusing on gamma scintigraphy, which involves noninvasive imaging of dosage forms to study their transit and absorption in vivo. It covers the properties and applications of radiopharmaceuticals, x-ray imaging, PET/SPECT, MRI, and ultrasonography in pharmaceutical formulation development and evaluation. Additionally, it addresses advantages and disadvantages of these imaging methodologies, alongside examples of practical applications in drug delivery systems.
![1
Mr. Sagar Kishor SavaleMr. Sagar Kishor Savale
[Department of Pharmaceutics][Department of Pharmaceutics]
avengersagar16@gmail.comavengersagar16@gmail.com
2015-20162015-2016](https://image.slidesharecdn.com/radioimagingtech-160529064604/85/Radio-Imaging-Techniques-1-320.jpg)