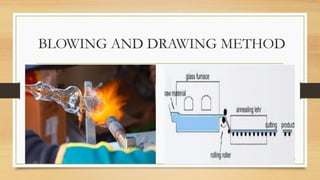
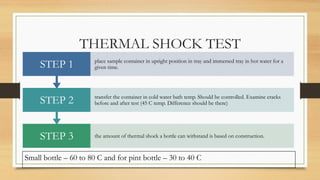
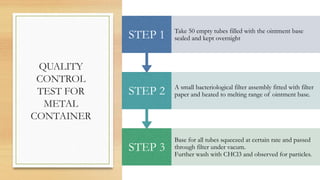
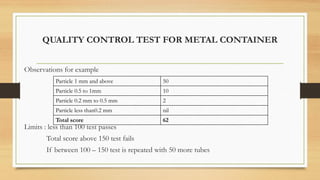
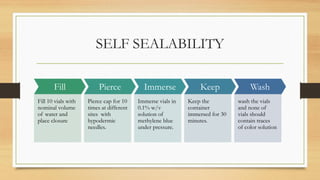
The document details the quality control processes and standards for packaging materials used in the pharmaceutical industry, emphasizing the importance of packaging in protecting products and ensuring compliance with testing standards. It categorizes packaging types into primary, secondary, and tertiary, and outlines the characteristics and materials used, including glass, plastics, metals, and rubber. Various quality control tests are described for ensuring the integrity, safety, and effectiveness of packaging, including methods for testing glass and plastic containers, as well as secondary packaging materials like cartons and papers.