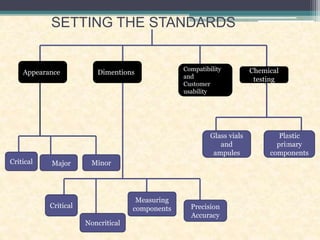
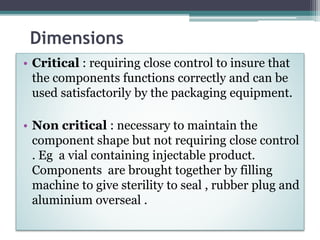
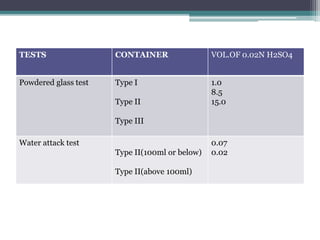
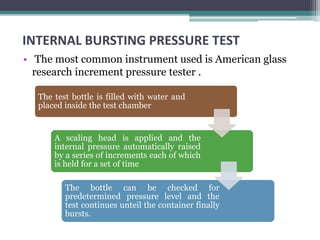
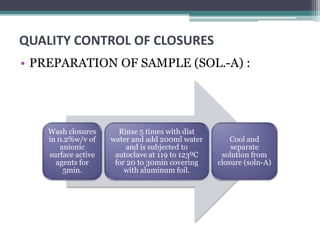
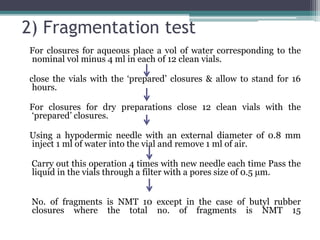
This document discusses quality control testing standards and procedures for pharmaceutical packaging materials. It outlines tests for various packaging components like glass containers, plastic packaging, and rubber stoppers. Key tests described include appearance and dimensional checks, compatibility testing, chemical resistance tests like powdered glass tests and water attack tests, sterility validation tests for sterile products, and non-sterile product validation tests. The document emphasizes that quality control testing is important to ensure packaging and components are defect-free and can safely contain drug products.