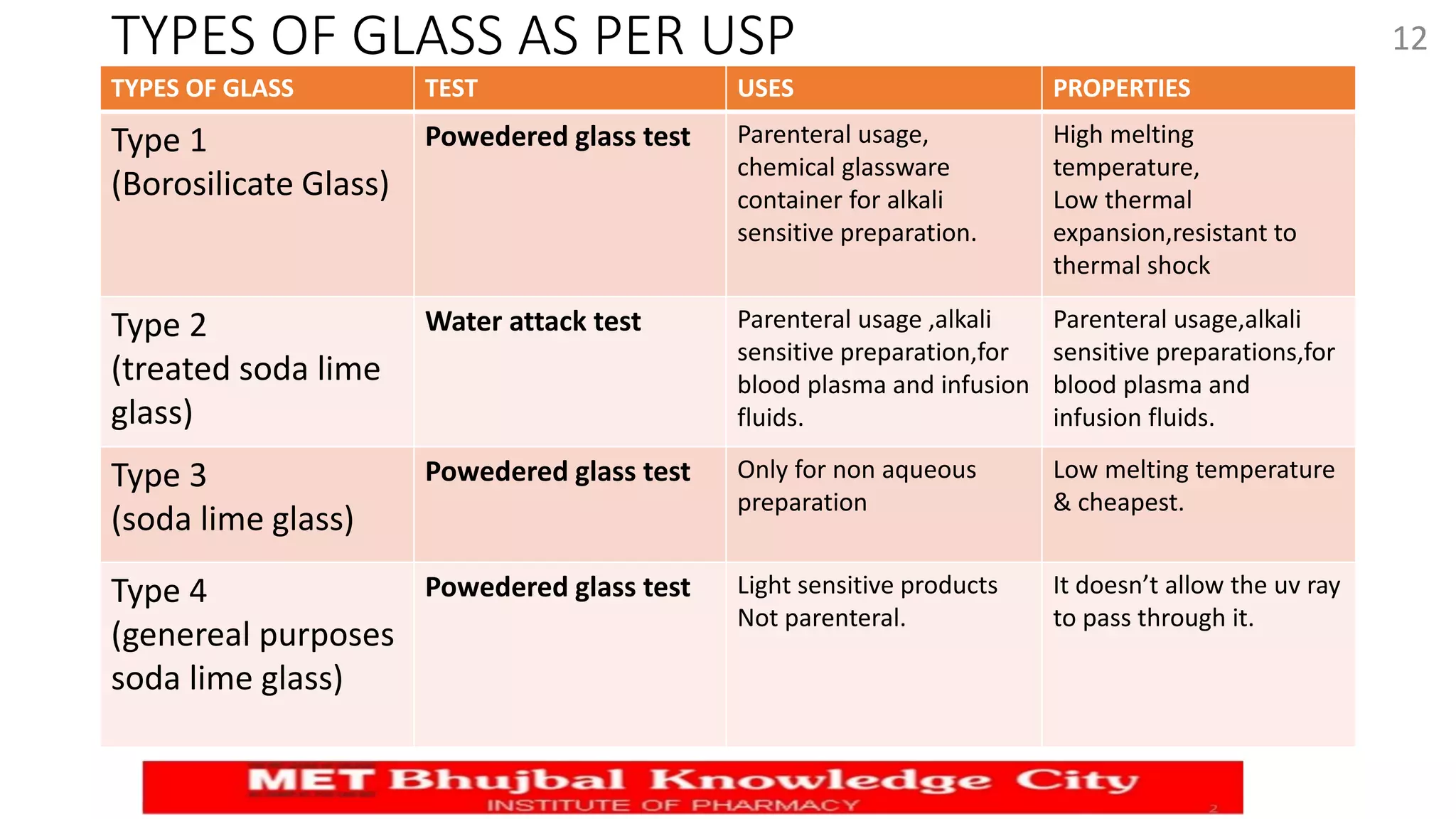
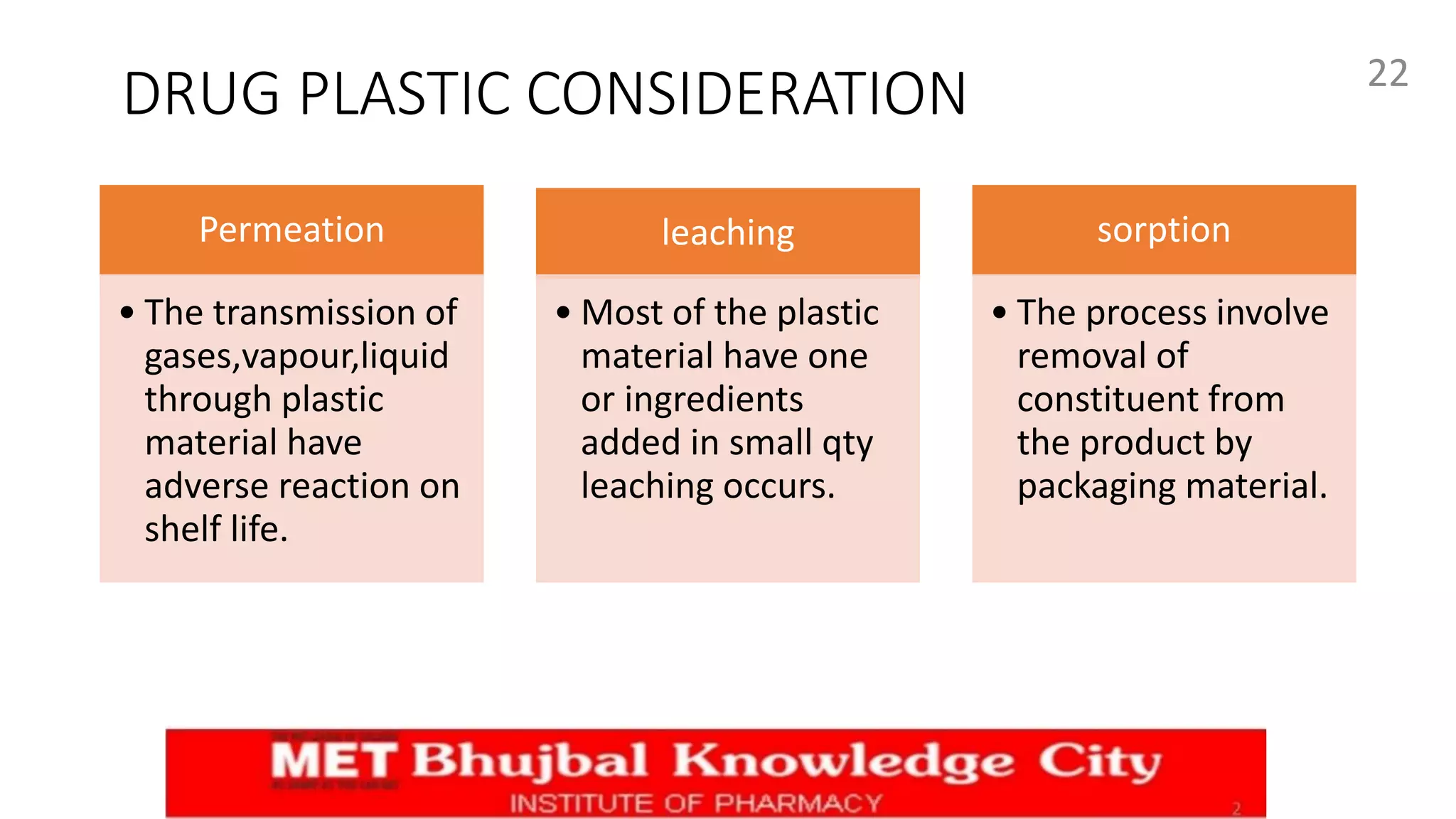
This document outlines key aspects of quality control tests for packaging materials in the pharmaceutical industry as part of a Master's seminar at Savitribai Phule Pune University. It covers the definitions, types, functions, and materials used in packaging, along with specific quality control tests for glass, plastic, metal, and rubber containers. Additionally, it discusses regulations such as tamper-resistant packaging and the importance of effective closure systems to maintain product integrity.