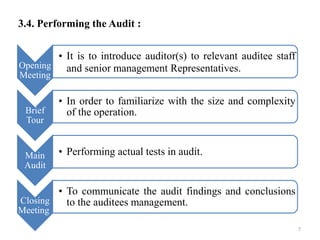
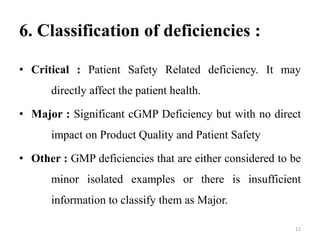
This document provides an introduction to auditing and outlines key aspects of conducting an audit. It discusses the types of audits, including internal and external audits. The objectives of audits are to ensure compliance with cGMP guidelines and facilitate continuous quality improvement. Planning and preparation are important aspects, such as determining the audit scope and composing an audit team. The audit process involves opening and closing meetings, a facility tour, performing tests, and reporting findings. An auditor's responsibilities include researching issues, ensuring corrective actions, and establishing effective control systems. Deficiencies are classified as critical, major, or other based on their impact.