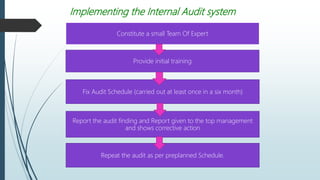
This document discusses audits in the pharmaceutical industry. It defines quality audits and GMP compliance audits. There are three main types of audits: internal audits conducted by a company on itself, external audits conducted by a company on its vendors, and regulatory audits conducted by bodies like the MCA and USFDA. Audits aim to verify compliance with regulations and allow for timely correction of problems. They help ensure quality systems are maintained and issues are identified and addressed. The roles of audits in quality assurance and quality control programs are also outlined.