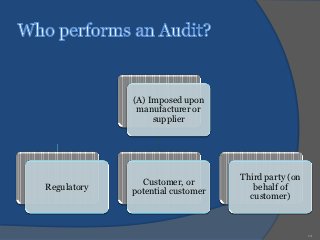
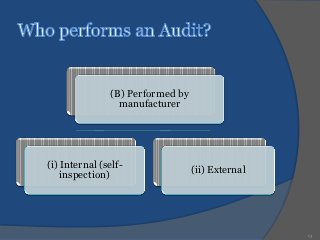
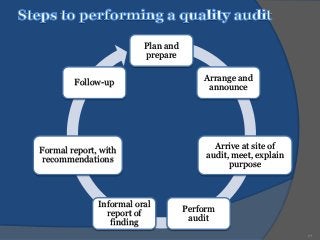
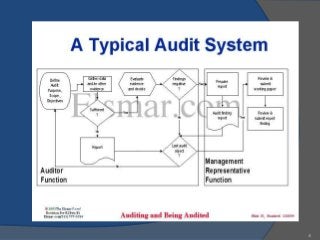
An audit is a systematic, independent examination to determine if quality activities and results comply with planned arrangements and if those arrangements are effectively implemented and suitable to achieve objectives. Audits are performed to collect objective evidence that allows for an informed judgment about the system or product being audited. There are internal audits performed by a company's own employees and external audits performed by outside parties like customers or independent organizations. The goal of audits is to determine compliance and ensure quality.