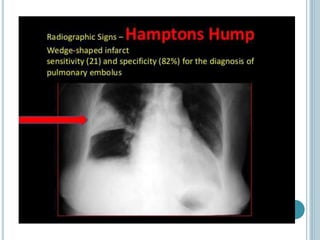
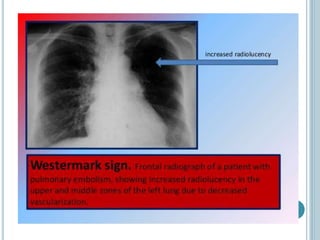
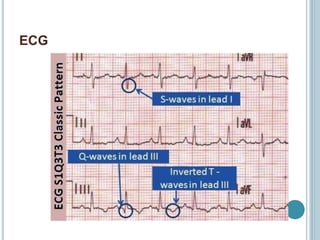
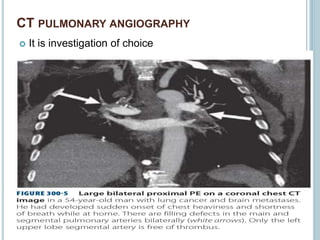
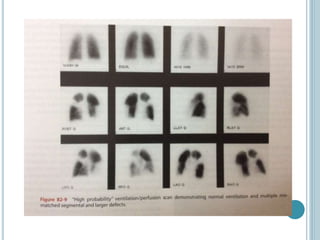
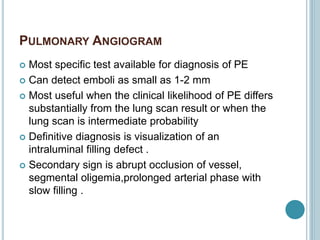
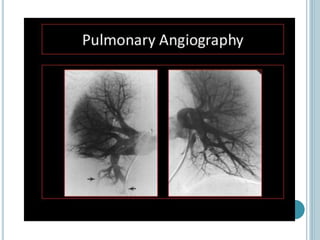
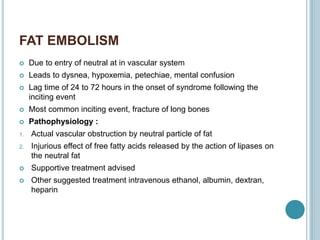
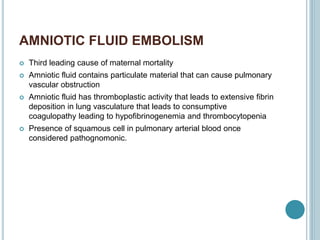
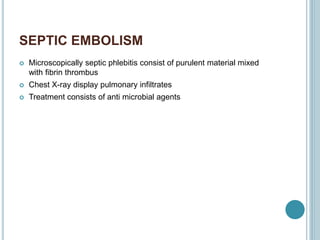
1. Pulmonary embolism is an obstruction of the pulmonary artery or its branches by a thrombus originating in the venous system or right side of the heart.
2. The annual incidence of PE ranges from 23-69 cases per 100,000 population in India. Globally, the incidence of venous thromboembolism remains relatively constant at 117 cases per 100,000 person-years.
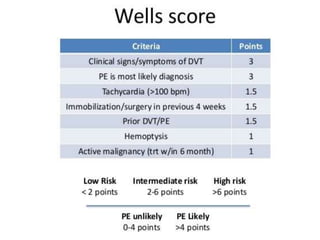
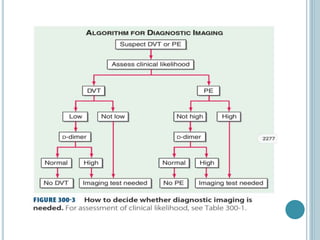
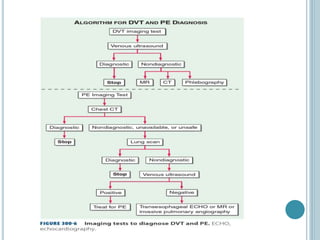
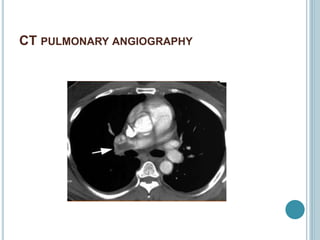
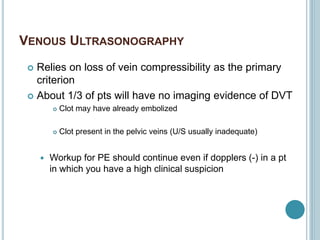
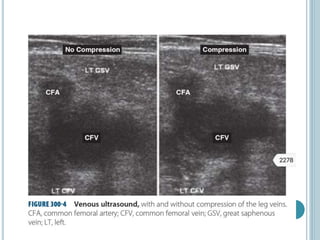
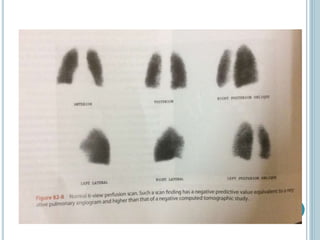
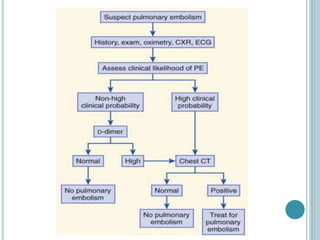
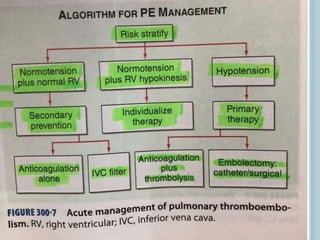
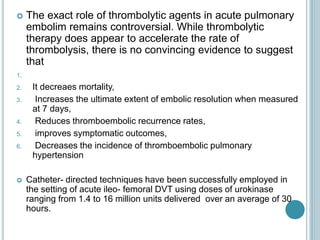
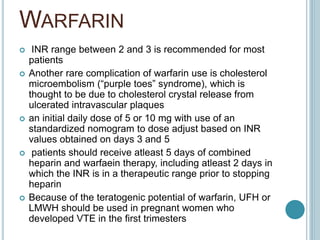
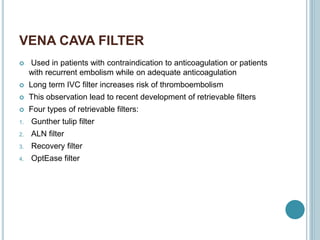
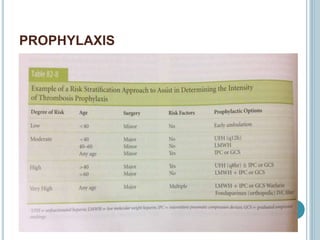
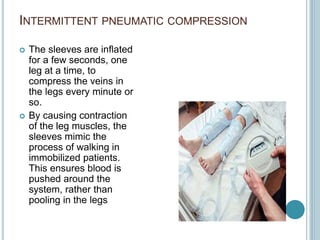
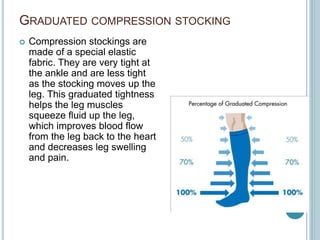
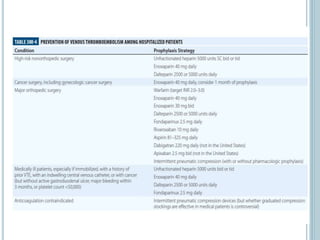
3. Diagnosis involves using criteria like Wells criteria and PERC rule to determine pre-test probability, D-dimer testing, and imaging like CT pulmonary angiography or lung scan if needed based on risk level and test results. Management involves anticoagulation with heparin or low molecular weight he