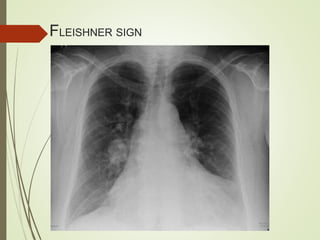
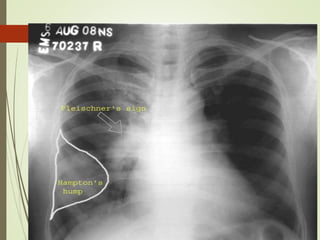
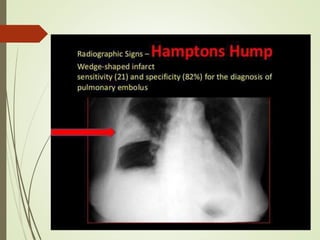
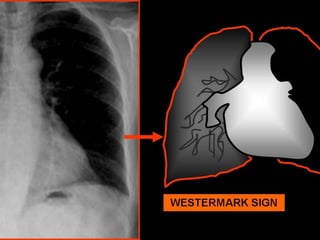
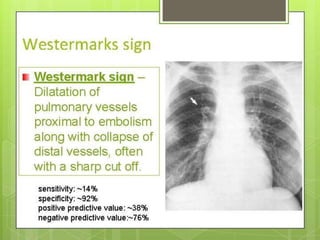
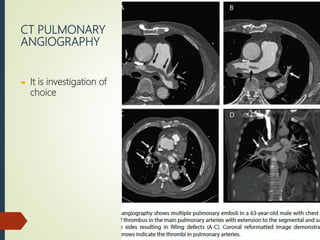
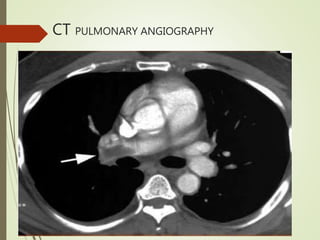
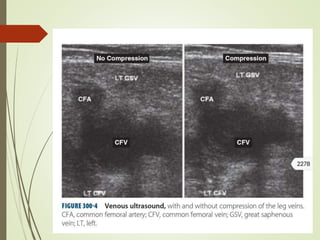
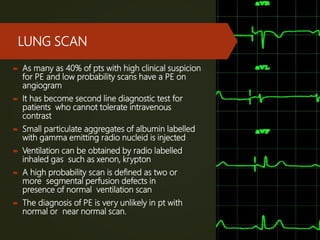
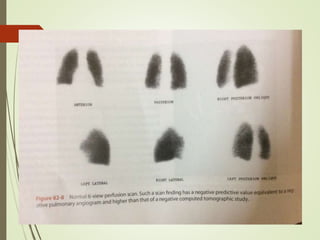
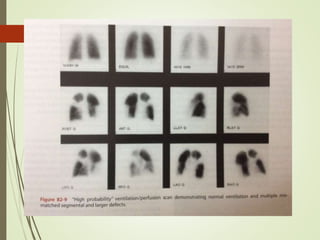
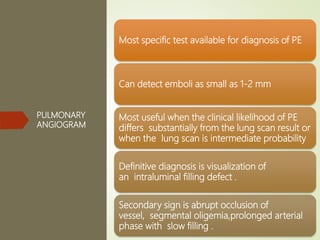
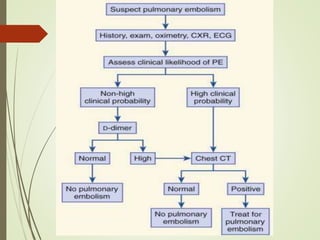
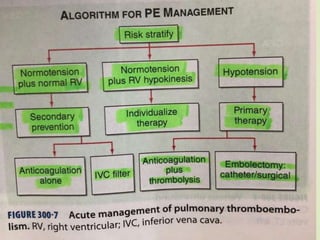
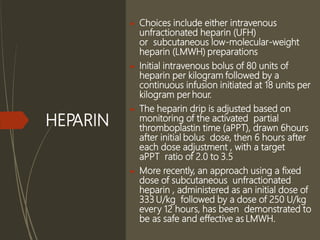
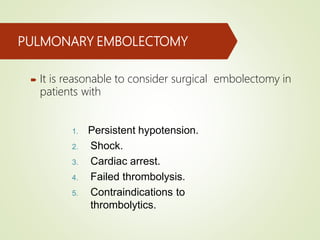
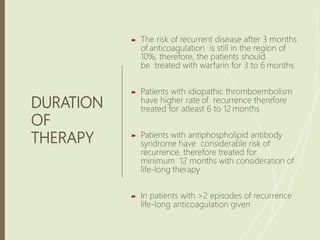
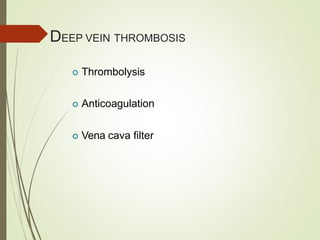
This document discusses pulmonary embolism (PE), including its definition, epidemiology, risk factors, pathophysiology, clinical features, diagnosis, and management. Some key points:
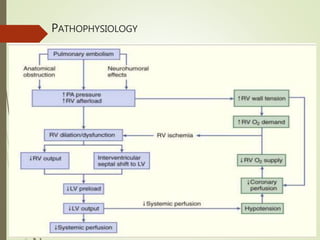
- PE is an obstruction of the pulmonary artery or its branches by a thrombus originating in the venous system or right heart. It is a common cause of preventable death in hospitalized patients.
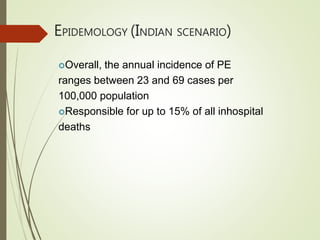
- The annual incidence of PE ranges from 23-69 cases per 100,000 people in India. Worldwide, the incidence of venous thromboembolism (which includes PE and DVT) is 117 cases per 100,000 person-years.
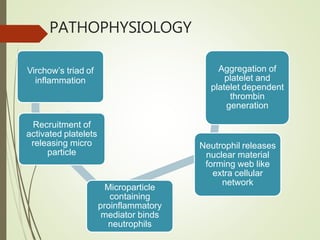
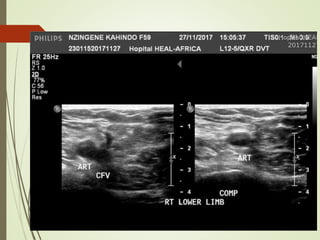
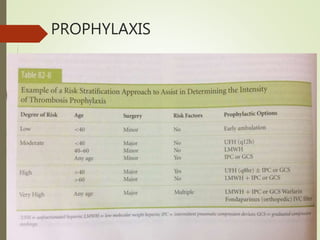
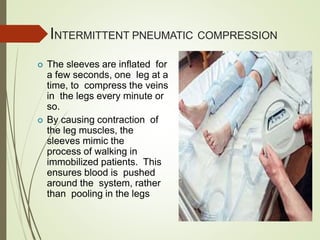
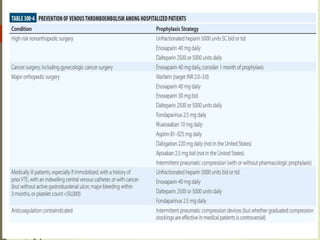
- Risk factors for PE include hereditary clotting disorders, immobil