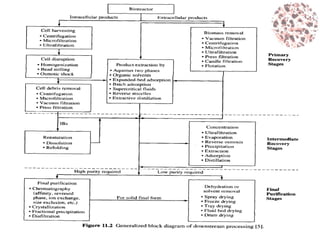
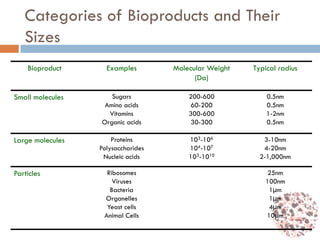
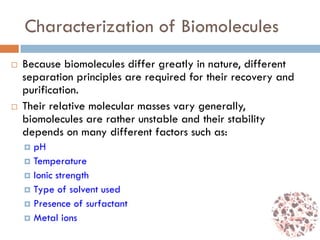
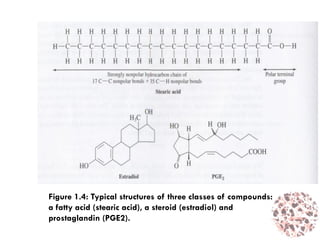
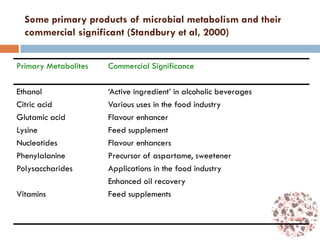
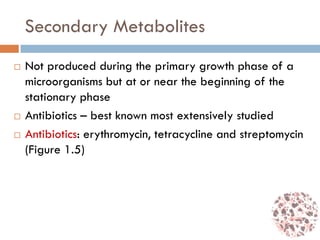
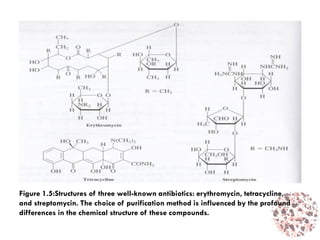
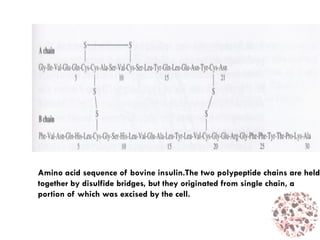
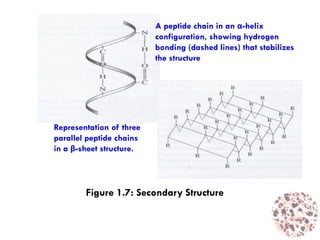
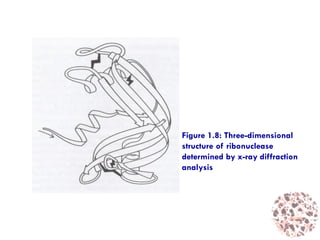
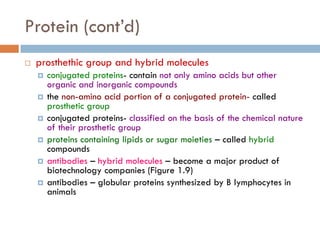
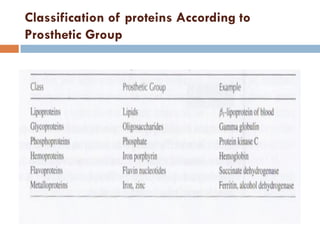
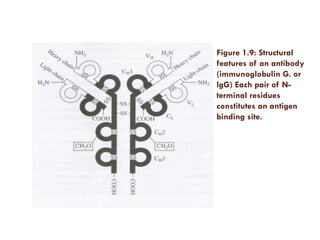
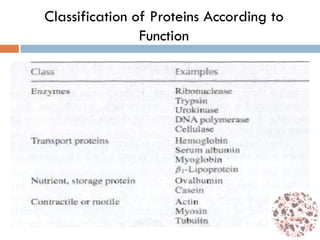
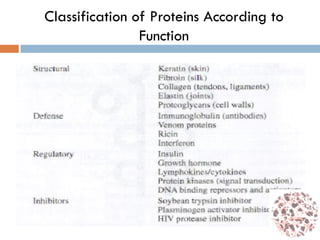
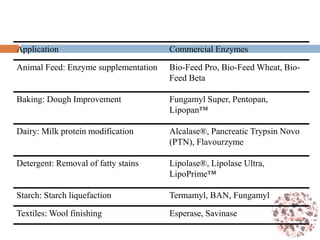
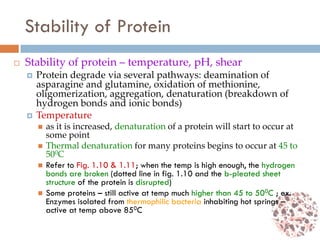
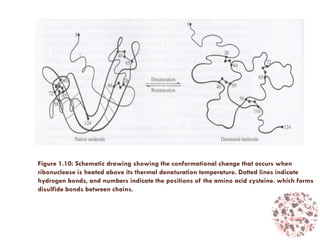
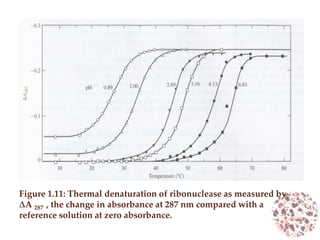
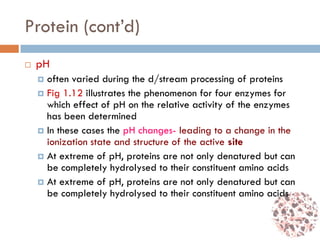
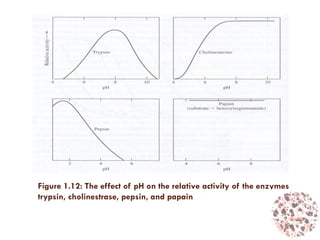
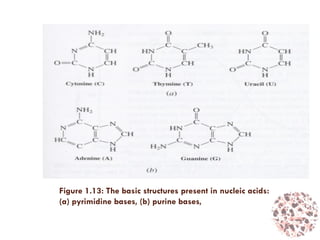
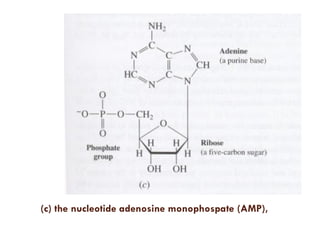
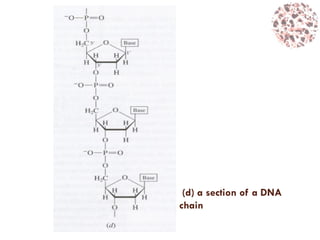
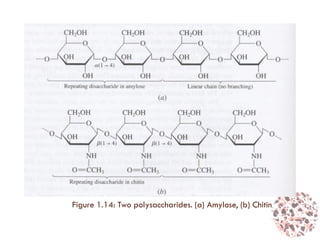
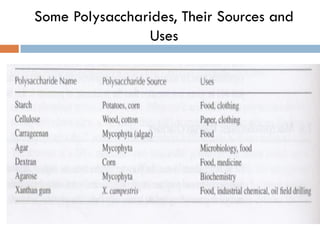
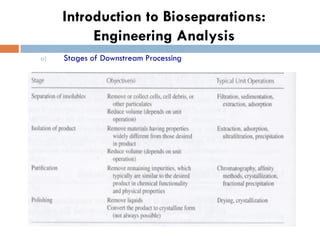
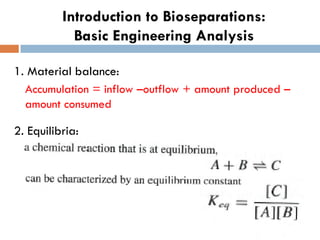
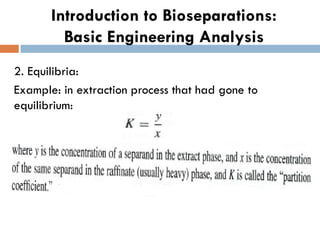
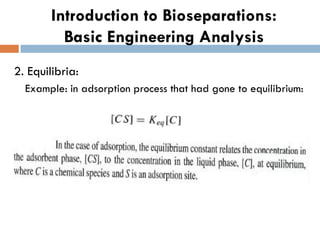
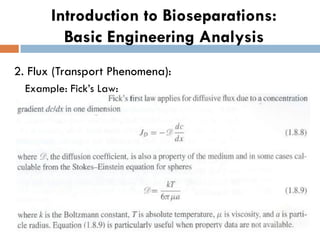
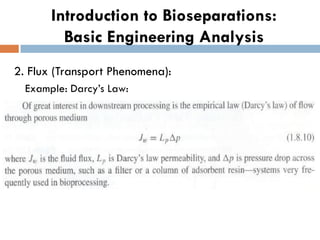
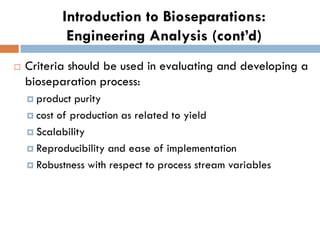
This document provides an introduction to bioproducts and bioseparations. It defines key terms like bioseparations and bioproducts. Bioproducts can come from whole cells, intracellular macromolecules, or extracellular products from microbial fermentations or cell culture. The choice of separation method depends on the nature and properties of the product. Bioproducts are sold based on their chemical activity. The design of large-scale purification requires defining the product, starting material properties, and possible separation steps. Common unit operations are discussed for each of the four stages of bioseparations. Characterization and stability considerations for different types of bioproducts like proteins, nucleic acids, polysaccharides, and particulate products