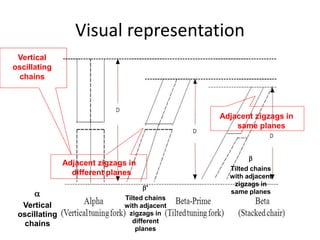
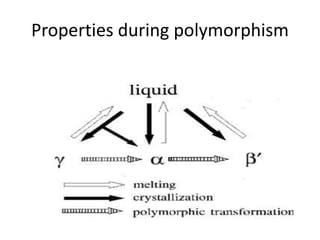
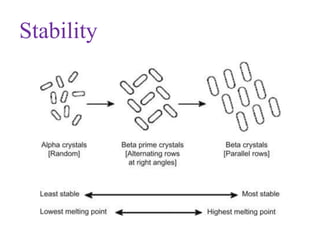
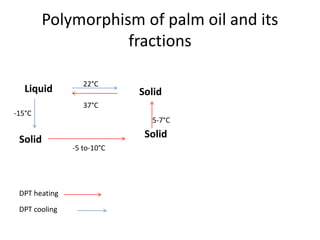
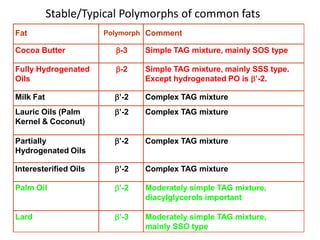
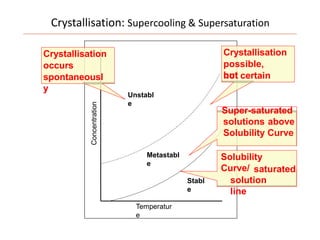
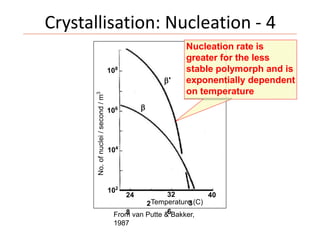
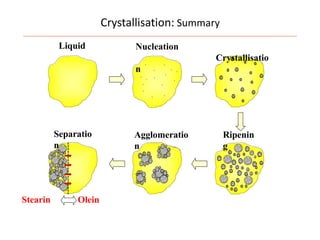
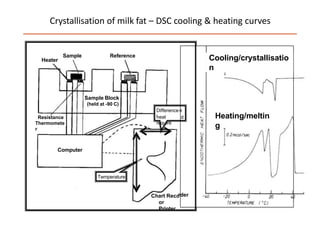
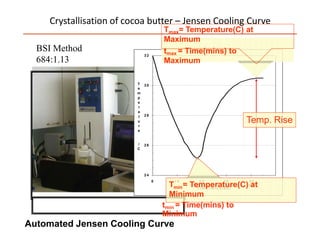
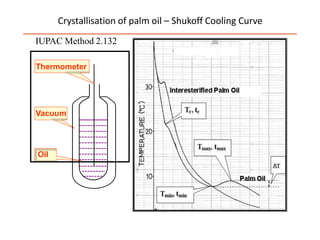
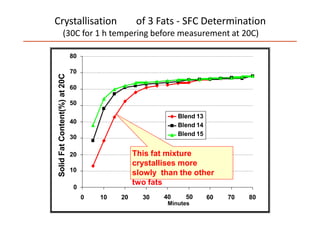
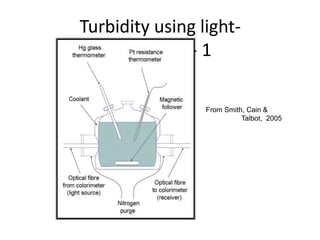
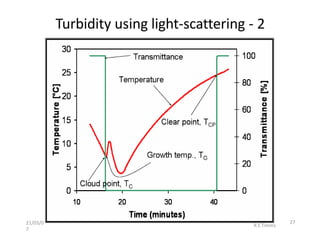
Polymorphism refers to the ability of a substance to exist in multiple crystalline forms. Fats and oils can exist in three main polymorphic forms - alpha, beta prime, and beta - with increasing stability. The transitions between forms occur with increasing temperature in the order of alpha to beta prime to beta. Methods for studying polymorphism include differential scanning calorimetry, which measures the heat of crystallization, and nuclear magnetic resonance spectroscopy, which determines the solid fat content during crystallization. Plasticity, the ability of a fat to be spread, arises from fats melting over a range of temperatures as different fatty acid components melt at different temperatures rather than at a single fixed point.