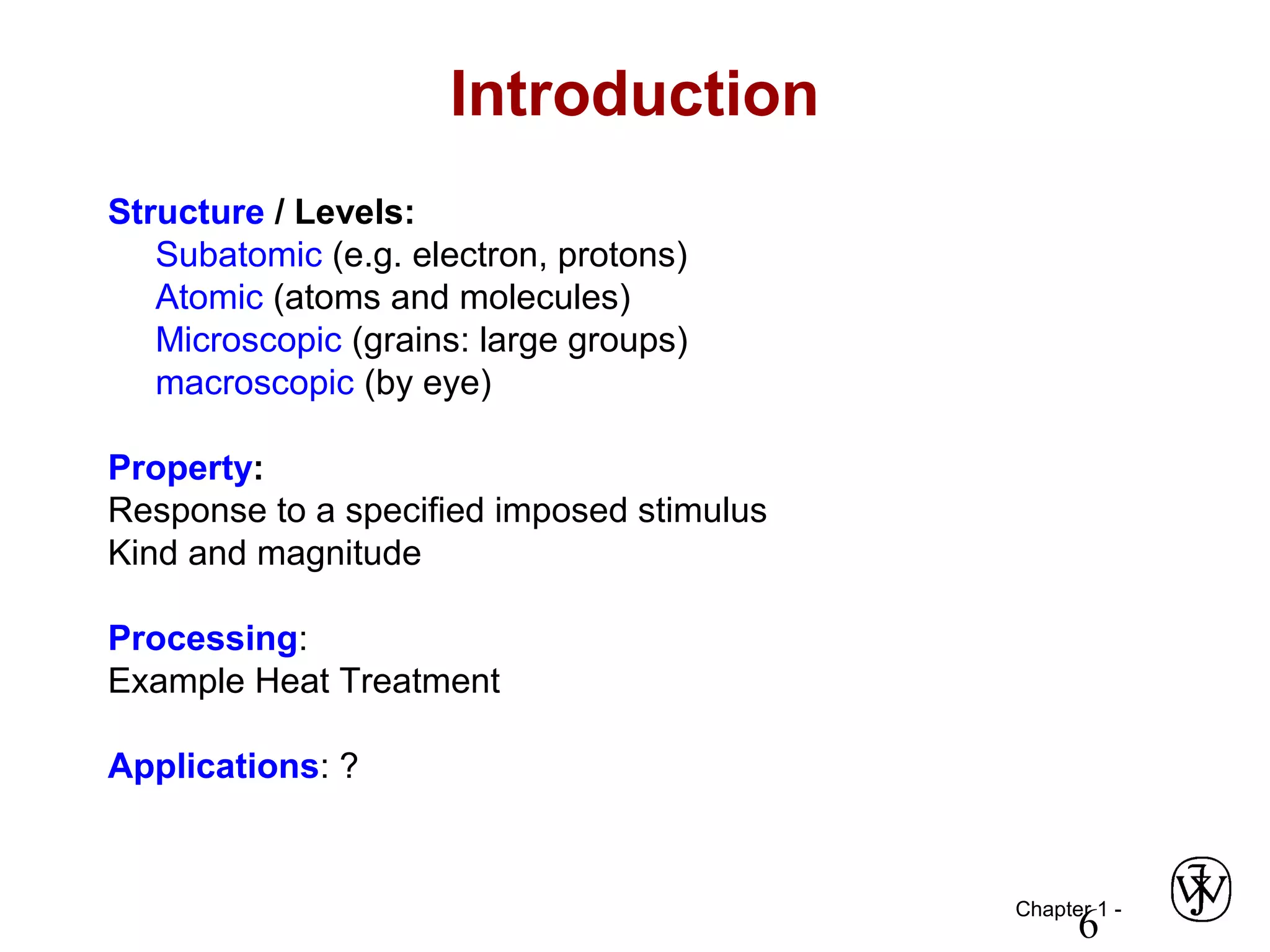
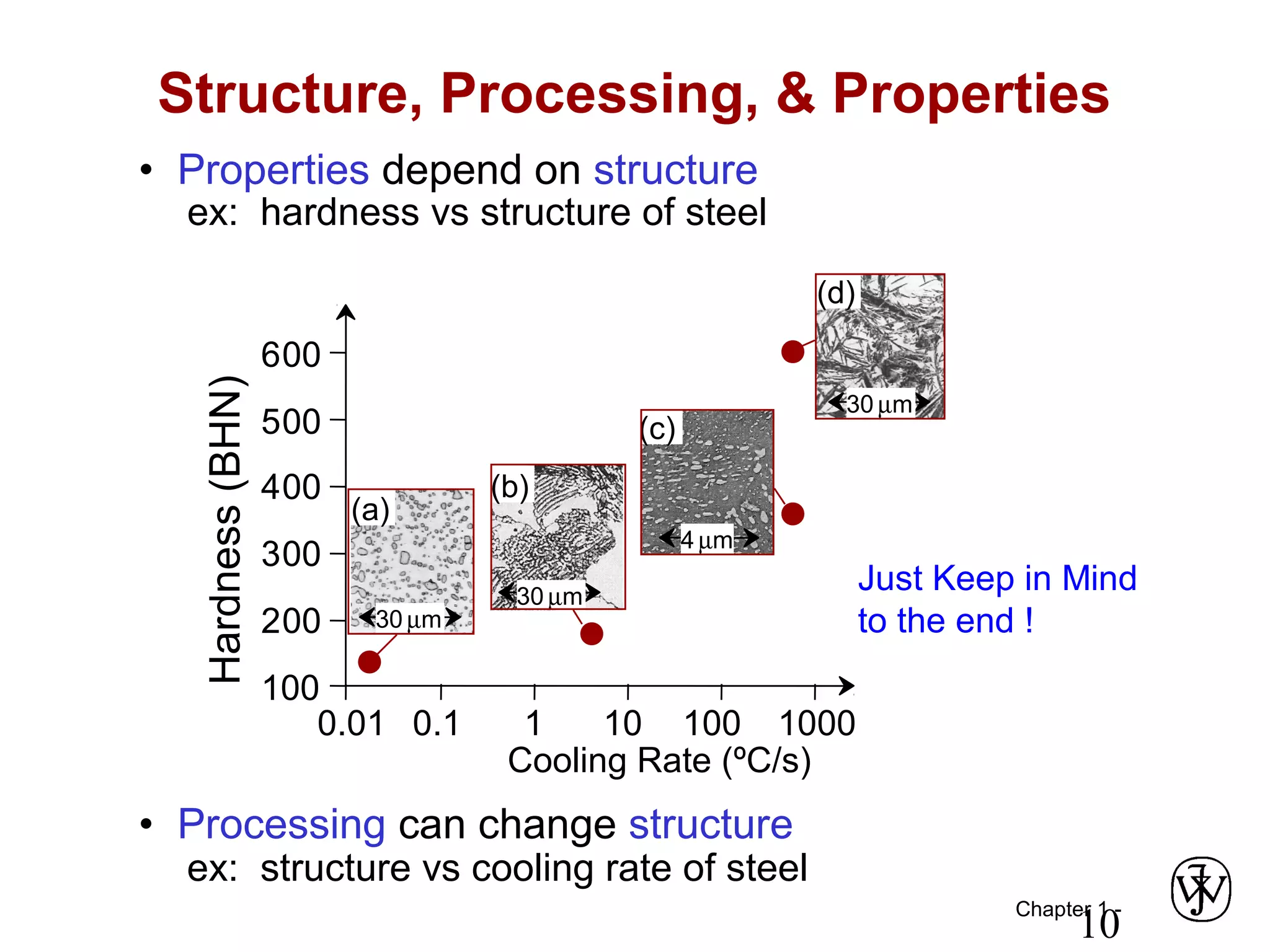
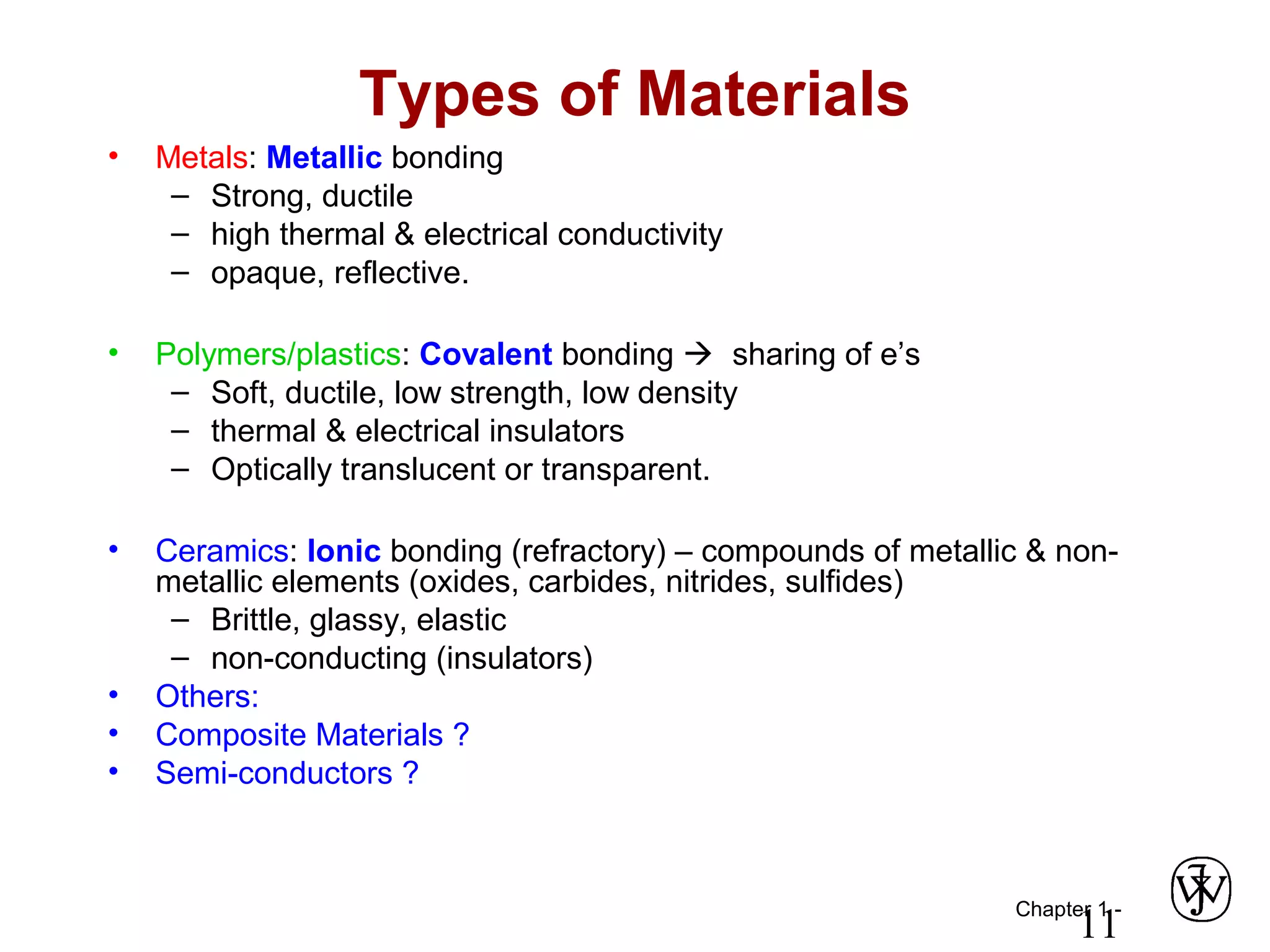
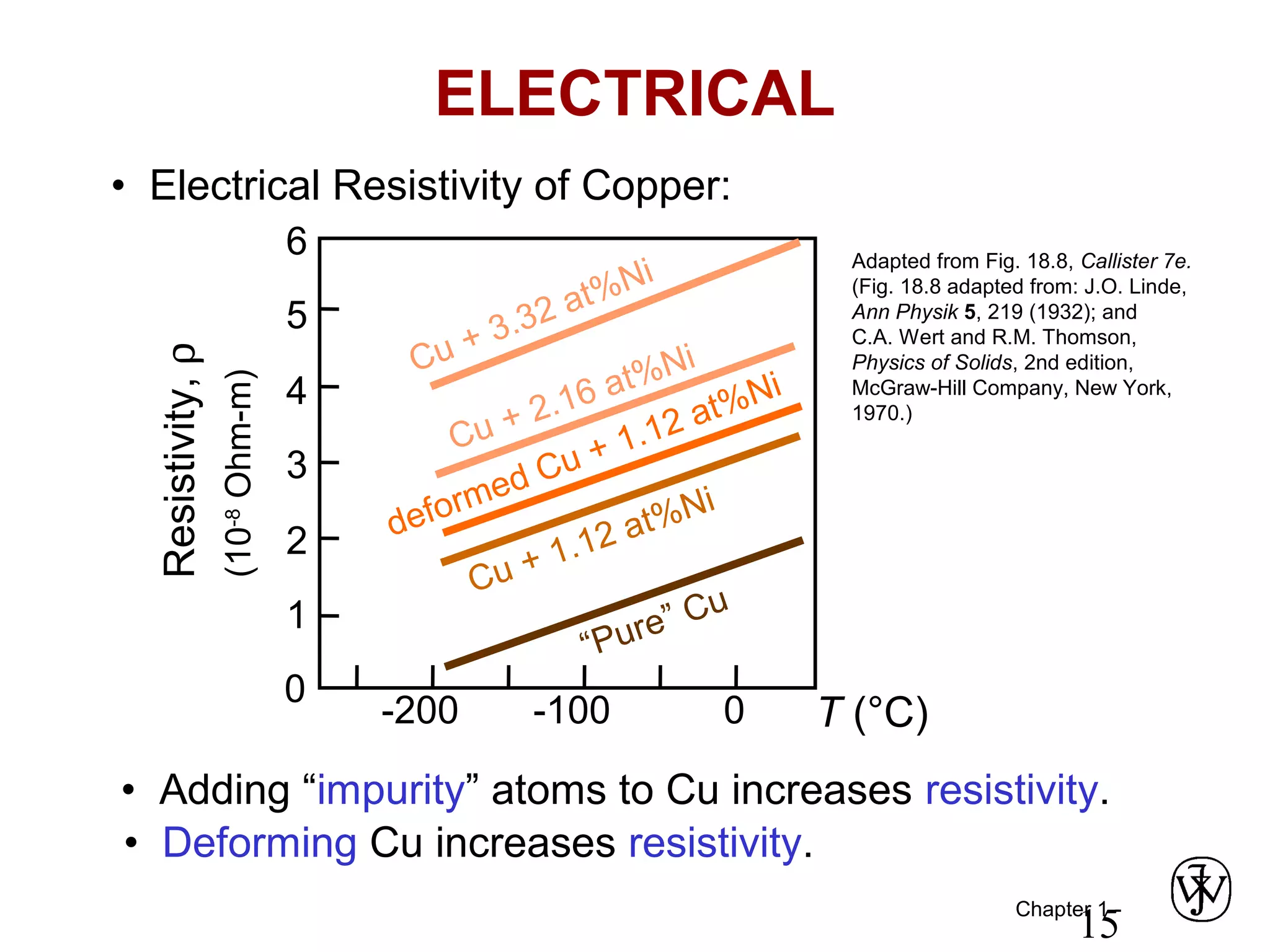
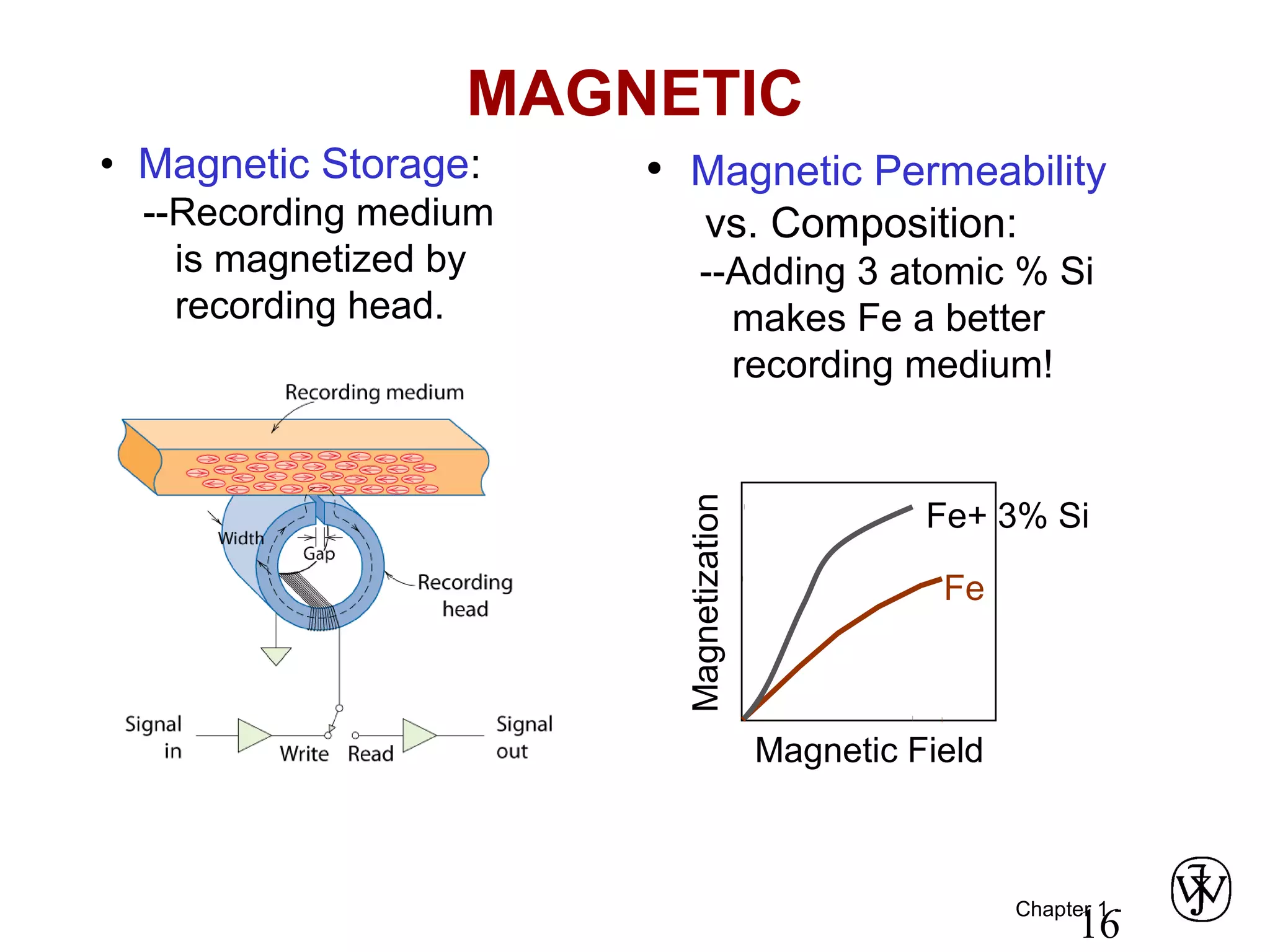
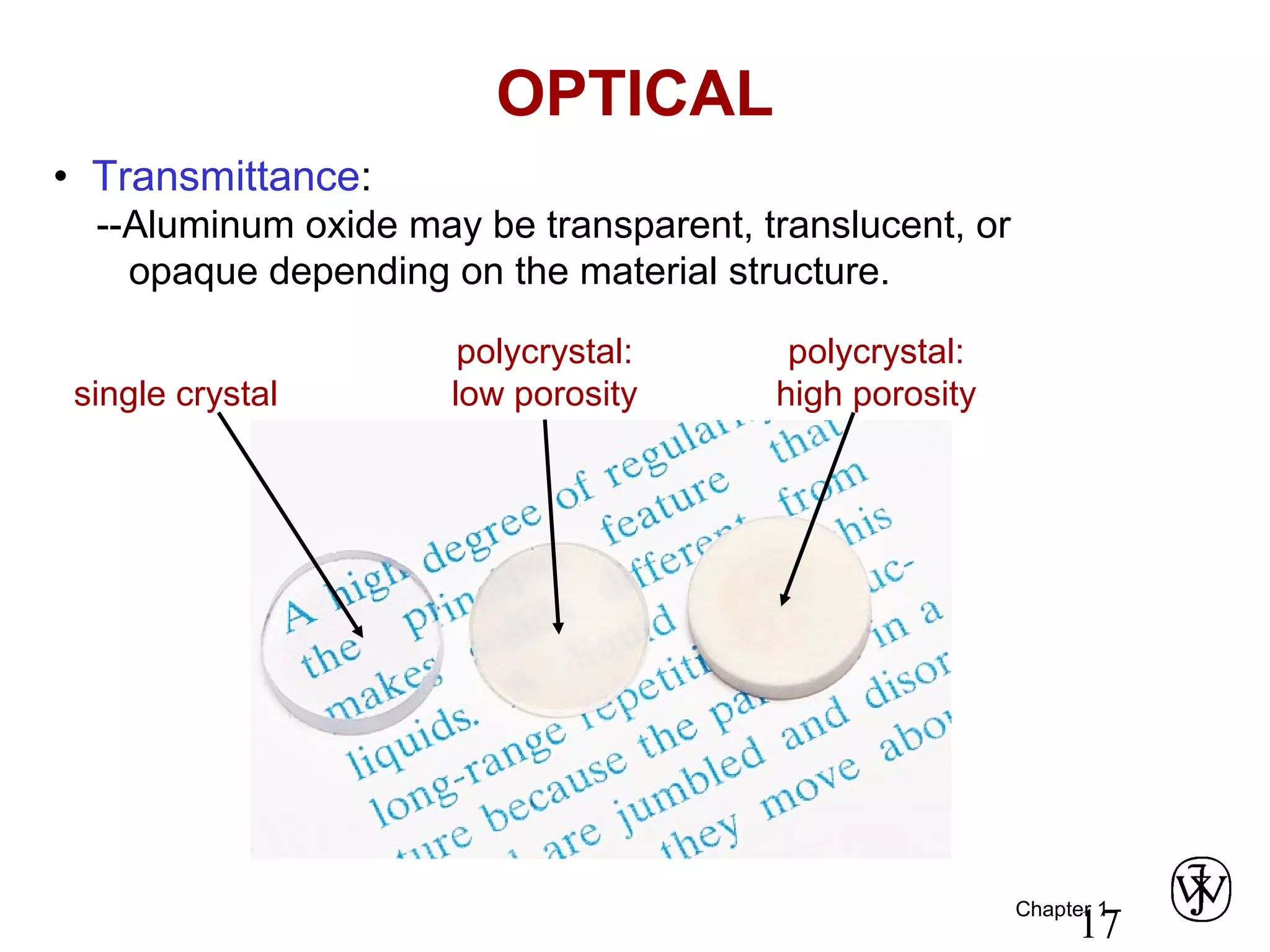
This document provides an overview of a Materials Science course, including the course objectives, topics, and policies. The course aims to introduce fundamental concepts of materials science including how material structure dictates properties and how processing can change structure. Over the semester, students will learn about various material types and properties, processing methods, and how the relationship between structure, processing and properties informs material selection for applications. The course will help students properly select materials, recognize new design opportunities, and understand the key relationship between processing, structure, and properties.