This document describes the synthesis of seven new analogues of the macrocyclic peptide sanguinamide B (SanB) and testing of their ability to inhibit protein synthesis in cancer cells. The analogues were designed by altering the amino acids at positions I and III of the SanB backbone, inverting stereochemistry at position III, and changing the protecting group on lysine. All analogues were tested for cytotoxicity against colon cancer cell lines and ability to inhibit protein synthesis. The lead compound with an IC50 of 15.9 μM against colon cancer cells contained an N6-carboxybenzyl-lysine at position I. This establishes the importance of this moiety for biological activity.

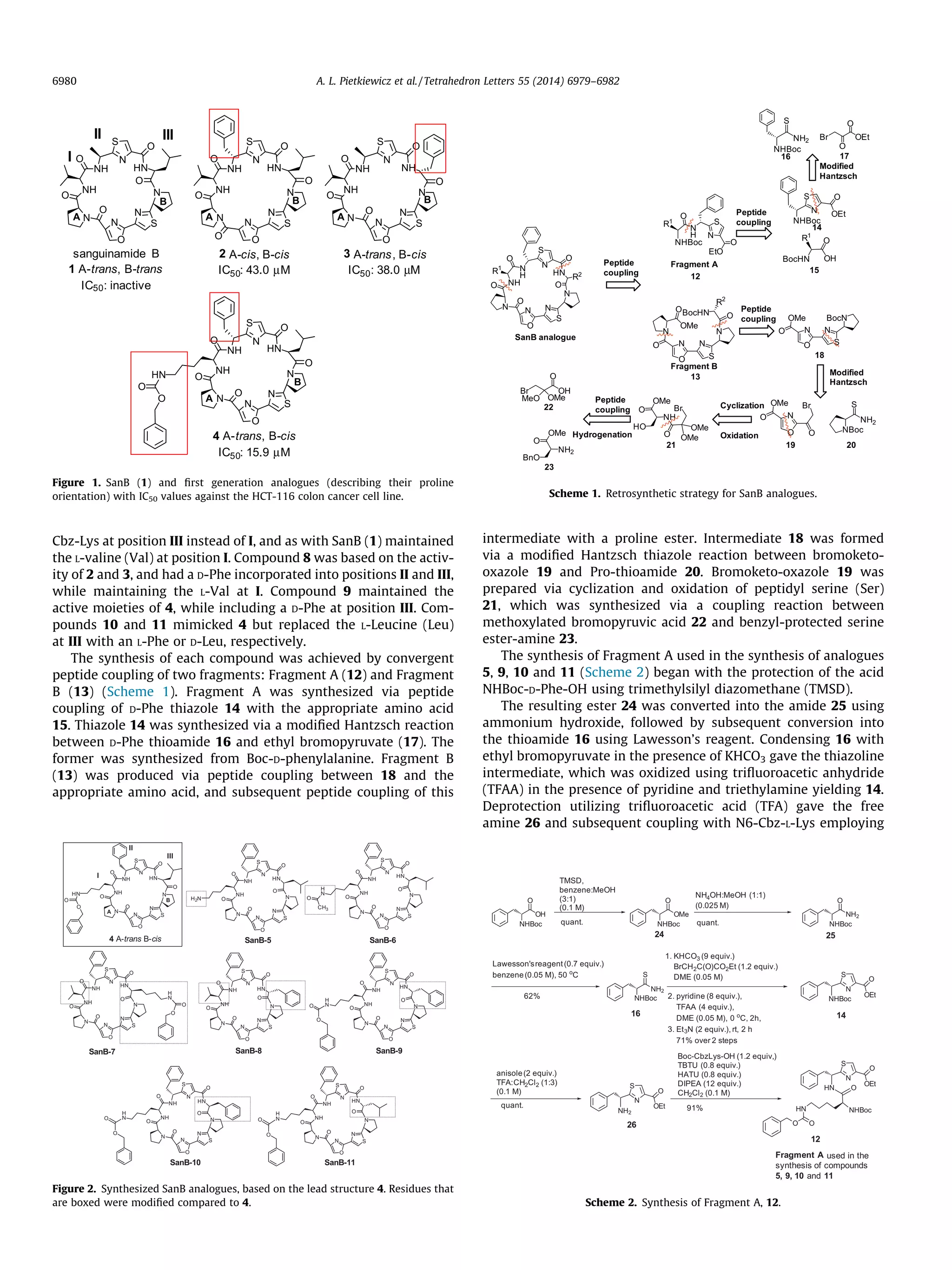
![TBTU and HATU as coupling agents, yielded Fragment A (12). Frag-
ment A required for synthesizing derivatives 7 and 8 had an L-Val
incorporated in place of Lys, and derivative 6 replaced the N6-Cbz-
L-Lys with an N6-Ac-L-Lys.
The synthesis of Fragment B (Scheme 3), 13, was achieved by
reacting Boc-Ser(Bn)-OH (27) with TMSD to generate the ester,
which was subsequently converted into the amine 23. A coupling
reaction between bromo-ketal acid 22 and NH2-Ser(Bn)-OMe (23)
yielded bromomethoxyketal serine derivative 28, which was sub-
jected to hydrogenation yielding compound 21. Cyclization of 21
using dimethylaminosulfur trifluoride (DAST), followed by oxida-
tion with bromotrichloromethane (BrCCl3) and 1,8-diazabicy-
clo[5.4.0]undec-7-ene (DBU) generated the oxazole 29. Ketone
deprotection of 29 using formic acid gave 19, which was reacted
with Pro-thioamide derivative 20 under modified Hantzsch thia-
zole conditions to give the bisheterocycle 18. Amine deprotection
of 18 with trifluoroacetic acid (TFA) and elongation by peptide cou-
pling with NHBoc-amino acid gave the ester 30, where coupling of
L-Leu, D-Leu, L-Phe, D-Phe or N6-Cbz-L-Lys generated the corre-
sponding Fragment B analogues for each derivative. Coupling
between 30 and NH-Pro-OMe yielded 13.
Coupling between the free amine and free acid, after respective
amine and acid deprotection reactions, yielded linear precursor 31
(Scheme 4). Subsequent acid and amine deprotection of 31, fol-
lowed by macrocyclization under dilute conditions generated all
the analogues, except 5. Compound 5 was generated by synthesiz-
ing compound 4 and removing the Cbz group from the lysine using
HBr and acetic acid (33% HBr in acetic acid) at 0.1 M.
Each analogue was purified by HPLC and LCMS, and the result-
ing NMR spectra showed that numerous analogues had more than
one conformation (Table 1). Identification of each conformer uti-
lized 1
H and 13
C NMR and 2D NMR experiments (1
H–1
H COSY,
1
H–13
C HSQC, 1
H–13
C HMBC), as well as HPLC, LC/MS, and HRMS
(see Supporting information).
Utilizing a gradation of temperatures in a 1
H NMR analysis pro-
vided the optimal temperature at which sharp peaks were
observed in the NMR.10
Upon determining the optimal tempera-
ture for evaluation, 2D NMR data were collected at that tempera-
ture, which allowed identification of the configuration of each
prolyl amide bond present in the macrocycle. Examination of the
Pro Cb and Cc chemical shifts provides evidence of the Pro being
‘cis’ or ‘trans’. A Pro amide bond that adopts cis orientation has a
larger DdCbc than a Pro with an amide bond in the trans orienta-
tion.13
The Pro Cb and Cc shifts for each conformer are shown in
Table 1.
From the data, it appeared that neither proline has a preferred
conformation. Although individual proline shifts were visible in
the 2D NMR data, which allowed us to assign the proline orienta-
tion, all but one compound (compound 11) were inseparable mix-
tures. As observed by isolating each peak of the inseparable
mixture on the LCMS and re-injecting into the LCMS, these mole-
cules were in fact oscillating between the two conformations. For
example, 6A and 6B were isolated as an interconverting mixture
of cis, cis and trans, cis, where prolyl A was rapidly oscillating
between cis and trans. Compound 8 was also isolated as two inter-
converting conformations with Pro A also oscillating between cis
and trans. Compounds 9 and 11 had Pro B oscillating between cis
and trans, where compound 11 interconverted slowly enough that
the two conformations could be isolated (Table 1).
The results of the biological testing show that the Cbz-Lys group
is essential for activity, since removal or modification of this resi-
DMTMM (1 equiv.),
HATU (1 equiv.),
DIPEA (12 equiv.)
CH2Cl2 (0.1 M)
O
N
S
N
O
N
O
R2
OEt
OS
N
NH
O
NH
N
O
BocHN
anisole (2 equiv.)
TFA:CH2Cl2 (1:3)
(0.1 M)
LiOH.H2O (8 equiv.)
H2O2 (3.4 equiv.)
MeOH (0.1 M)
quant.
quant.
R1
Fragment B
13
O
N
S
N
O
N
O
R2
OEt
OS
N
NH
O
NHBoc
N
O
BocHN
R1
OMe
O
N S
NO
N
O
HN
OS
N
NH
O
NH
R1
N
O
1. LiOH.H2O (8 equiv.)
EtOH (0.1 M)
2. anisole (2 equiv.)
TFA:CH2Cl2 (1:3)
(0.1 M)
3. HATU (1 equiv.)
DMTMM (1 equiv.)
T3P (1 equiv.)
DIPEA (12 equiv.)
CH2Cl2 (0.0005 M)
10-79% over 3 steps
* for compound 5 HBr/AcOH (33%) 0.1 M
was added to 4 which removed the Cbz group
SanB analogue
31
Fragment A
12
Yields ranged from
33-98% over 3 steps
R2
R1 =
NH2
H
N CH3
O
NHCbz
R2 =
NHCbz
A
B
Scheme 4. Cyclization and synthesis of SanB analogues.
22 (1.2 equiv.)
TBTU (1 equiv.)
HATU (1 equiv.)
DIPEA (12 equiv.)
CH2Cl2 (0.1 M)
OMe
O
BnO
NH
O
OMe
Br
OMe
formic acid (0.1 M)
rt to 60 o
C
O
N
O
O
OMe Br
S
N
BocN
N
O
O
OMe
H2, Pd/C (10% w/w)
EtOH (0.1 M)
OMe
O
HO
NH
O
OMe
Br
OMe
1. DAST (2 equiv.)
K2CO3 (2 equiv.)
-78 o
C to rt
CH2Cl2 (0.1 M)
2. DBU (2 equiv.),
BrCCl3 (2 equiv.)
-46 o
C to rt
CH2Cl2 (0.1 M)
OMe
N
O
OMe Br
OMe
93%
OH
O
BnO
NH
Boc
1. TMSD
MeOH:benzene
(1:3)
(0.1 M)
2. anisole (2 equiv.)
TFA:CH2Cl2 (1:3)
(0.1 M)
OMe
O
BnO
NH2
O
1. KHCO3 (8 equiv.)
20 (1.2 equiv.)
DME (0.05 M)
2. pyridine (9 equiv.),
TFAA (4 equiv.),
DME (0.05 M)
0 o
C, 2 h,
3. Et3N (2 equiv.),
0 oC to rt, 2 h,
72% over 3 steps
quant. for 2 steps
83% quant.
75% over 2 steps
1. anisole (2 equiv.)
TFA:CH2Cl2 (1:3)
(0.1 M)
quant.
2. NHBoc-Amino Acid-OH (1.2
equiv.)
TBTU (1 equiv.)
HATU (1 equiv.)
DIPEA (12 equiv.)
CH2Cl2 (0.1 M)
84% over 2 steps
OMe
S
N
N
N
O
O
O
R2
BocHN
1. LiOH (8 equiv.)
MeOH (0.1 M)
N
S
N
N
N
O
O
O
R2
BocHN
O
OMe
2. NH-Pro-OMe (1.2 equiv.)
TBTU (1 equiv,)
HATU (1 equiv.)
DIPEA (12 equiv.)
CH2Cl2 (0.1 M)
74% over 2 steps
Fragment B
2327 28
21 29 19
18
30
13
Scheme 3. Synthesis of Fragment B, 13.
Table 1
Conformational assignment of SanB-14 (4) and its analogues 6–11. Orientation of the
prolyl amide bond is determined by DdCbc.13
(Data for SanB-4 were referenced from
the published result.12
Compound Conformer DdCbc13
Pro A DdCbc Pro B Assignment
SanB-412
5.7 9.5 trans, cis-
SanB-6 A 9.5 15.0 cis, cis
B 4.4 10.9 trans, cis
SanB-7 7.5 14.2 trans, cis
SanB-8 A 7.0 7.7 trans, trans
B 9.6 2.5 cis, trans
SanB-9 A 4.3 8.6 trans, cis
B 6.6 5.0 trans, trans
SanB-10 9.3 14.7 cis, cis
SanB-11 A 9.9 14.0 cis, cis
B 9.7 7.8 cis, trans
(see Supporting information for details on structure conformation.)
A. L. Pietkiewicz et al. / Tetrahedron Letters 55 (2014) 6979–6982 6981](https://image.slidesharecdn.com/d18061d4-4c63-4438-a0a8-a93b7b870de8-150923074324-lva1-app6892/75/2014-Pietkiewicz-Wahyudi-Synthesis-of-macrocycles-that-inhibit-protein-synthesis-SanB-3-2048.jpg)
