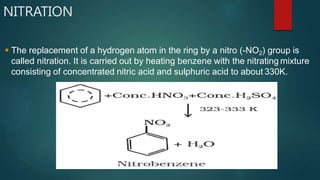
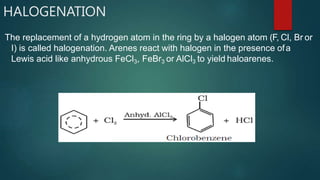
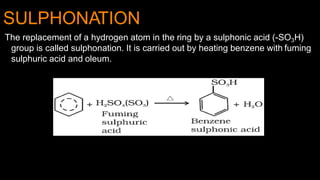
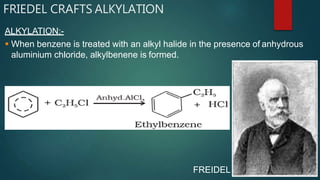
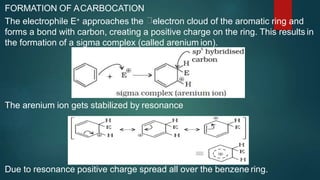
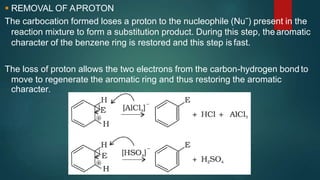
Electrophilic substitution reactions involve replacing a hydrogen atom in an aromatic ring with an electrophilic group. Nitration replaces hydrogen with a nitro group using nitric and sulfuric acid. Halogenation uses a halogen and Lewis acid. Sulphonation uses fuming sulfuric acid and oleum. Friedel-Crafts alkylation and acylation use an alkyl or acyl halide with aluminum chloride to add those groups. The mechanism involves generating an electrophile, forming a carbocation intermediate, and removing a proton. Ortho and para directing groups increase electron density, favoring substitution at those positions, while meta directing groups decrease electron density, favoring meta position. Polynuclear hydrocarbons from