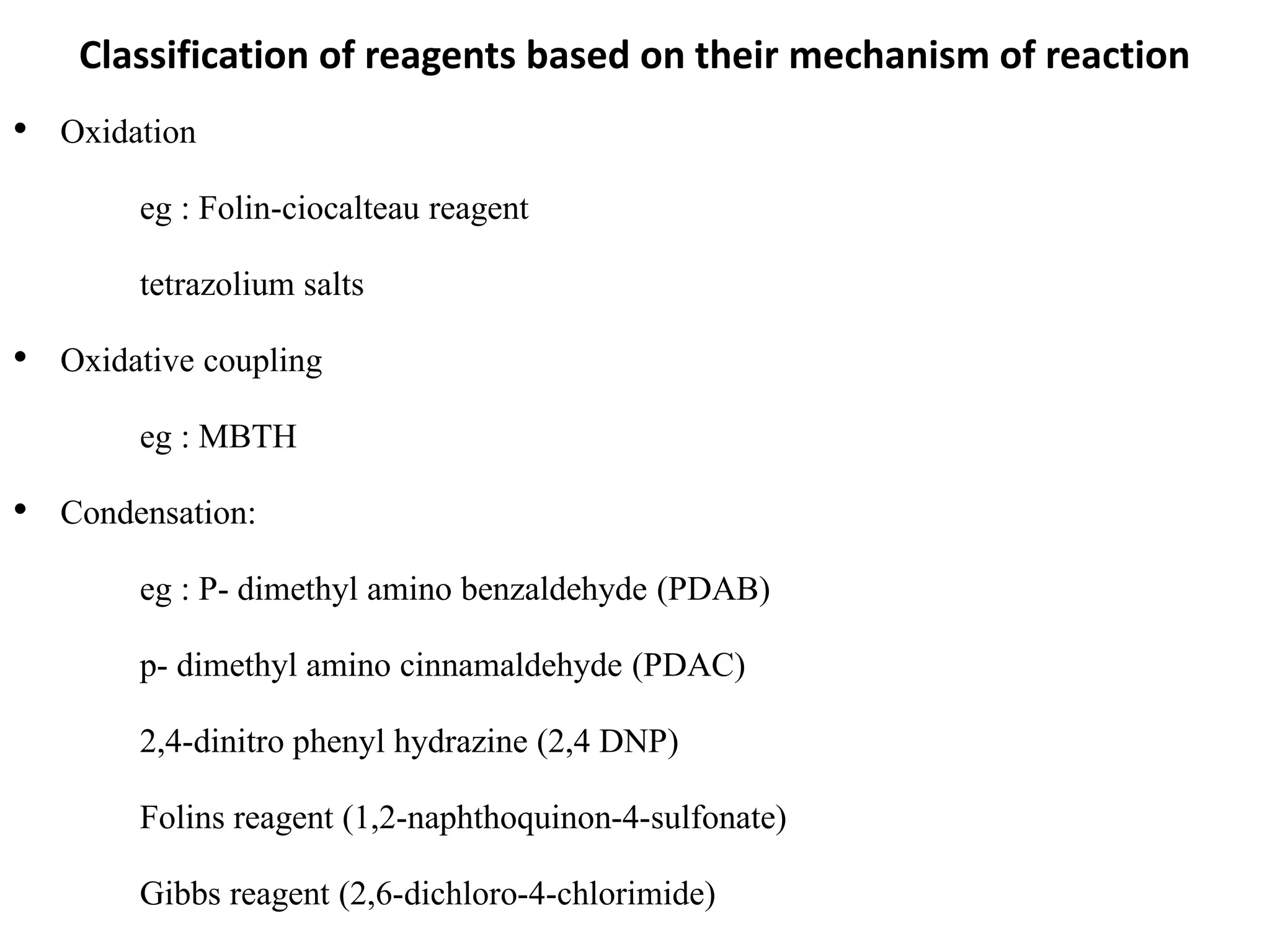
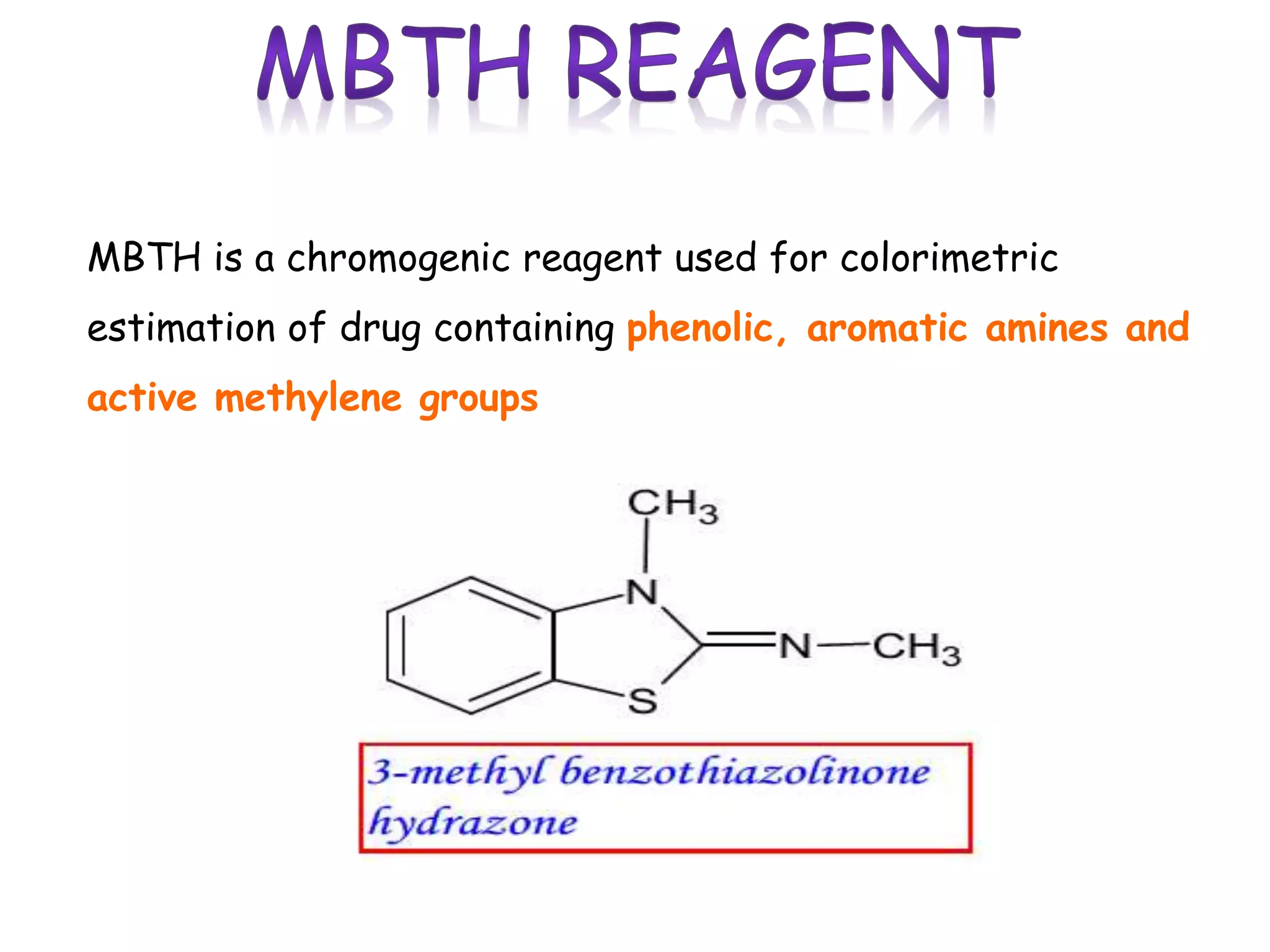
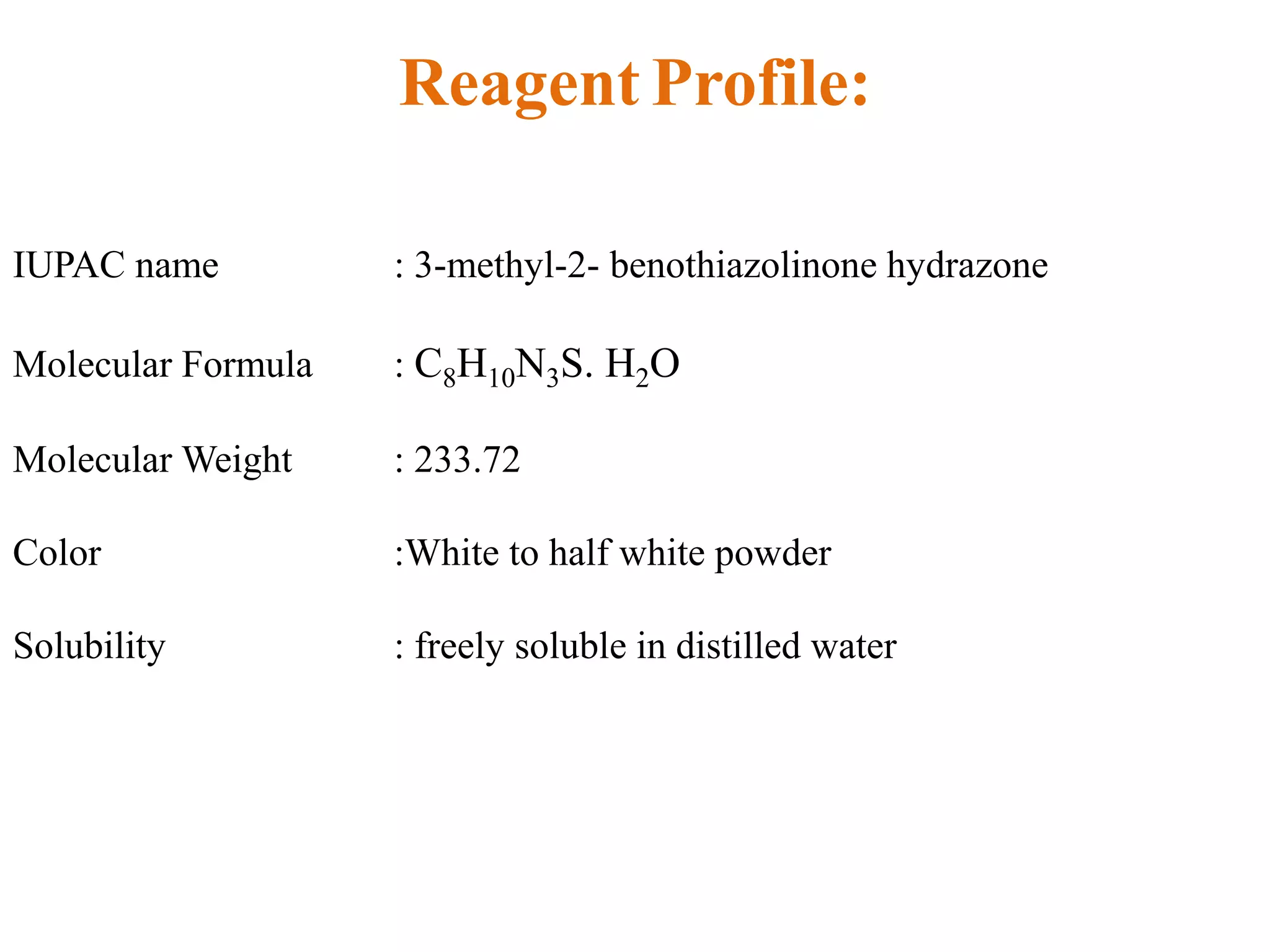
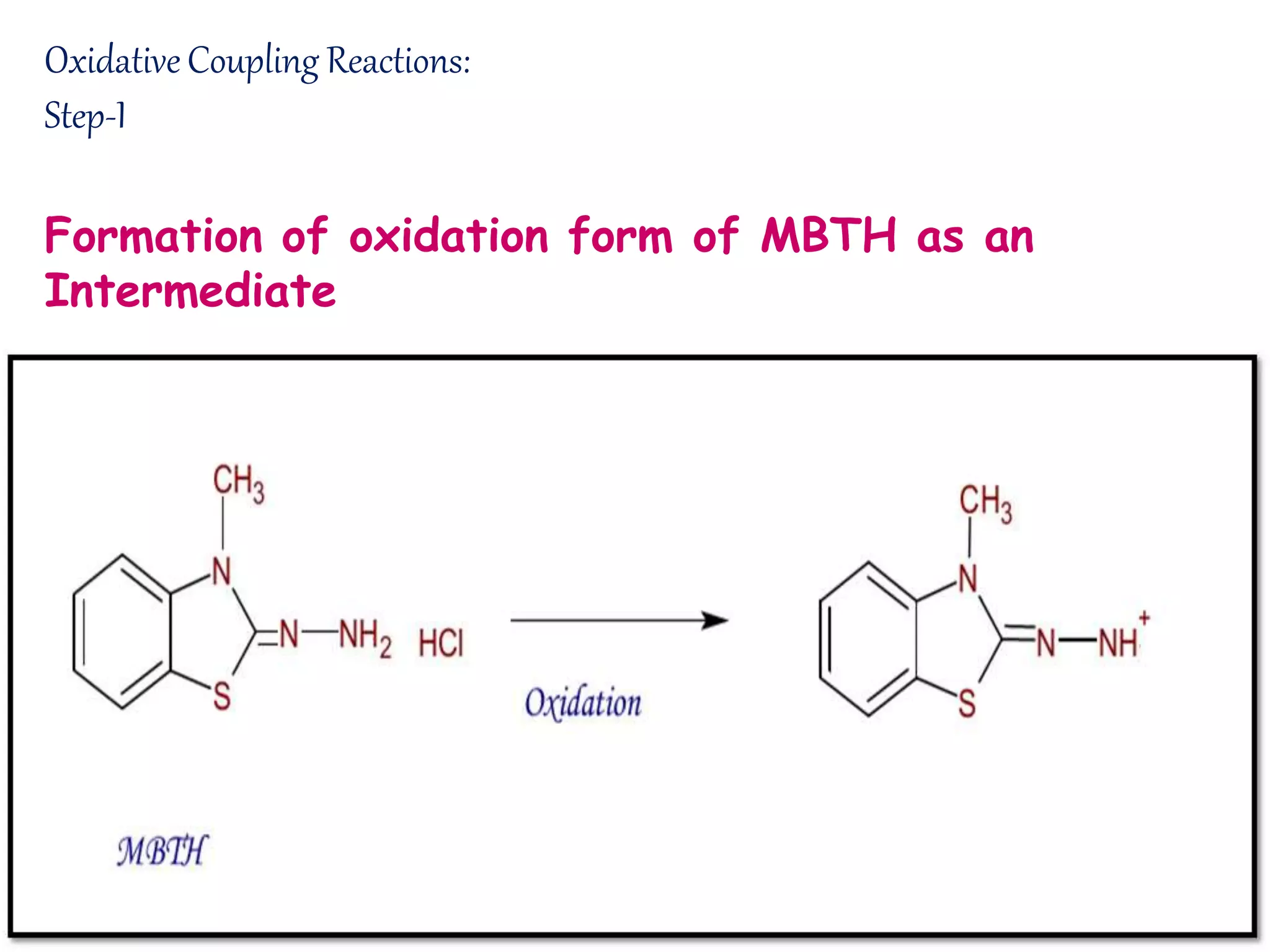
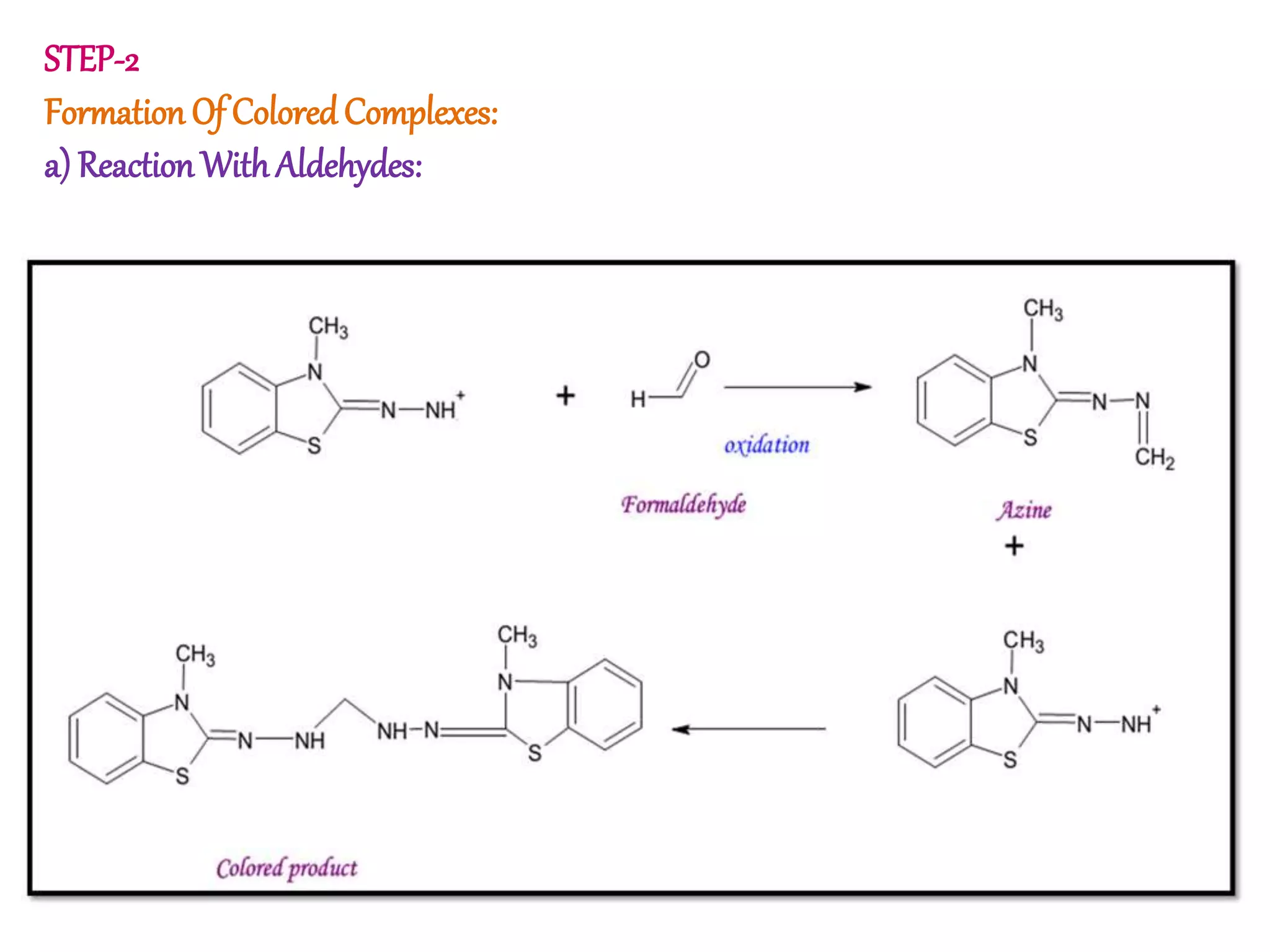
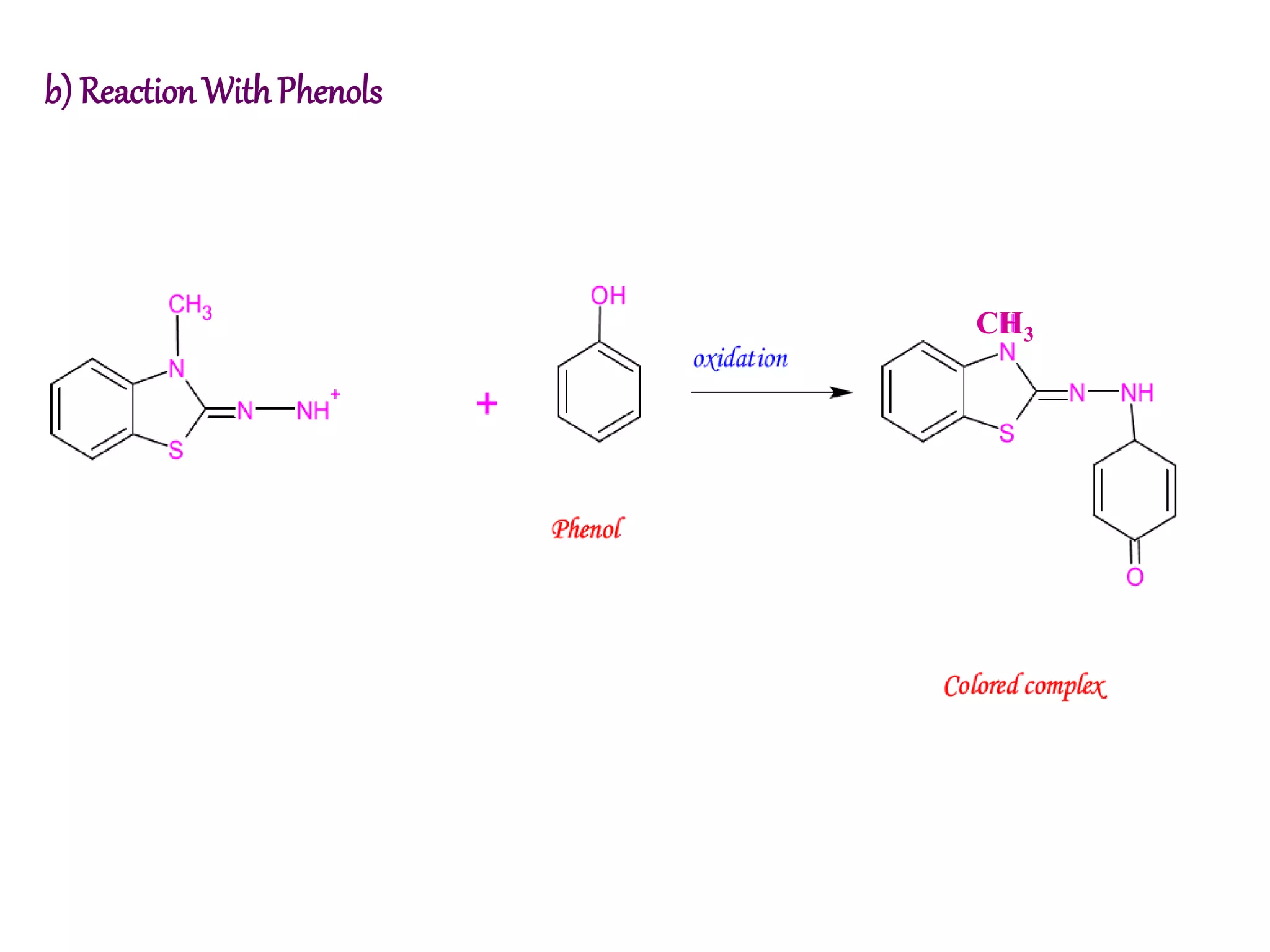
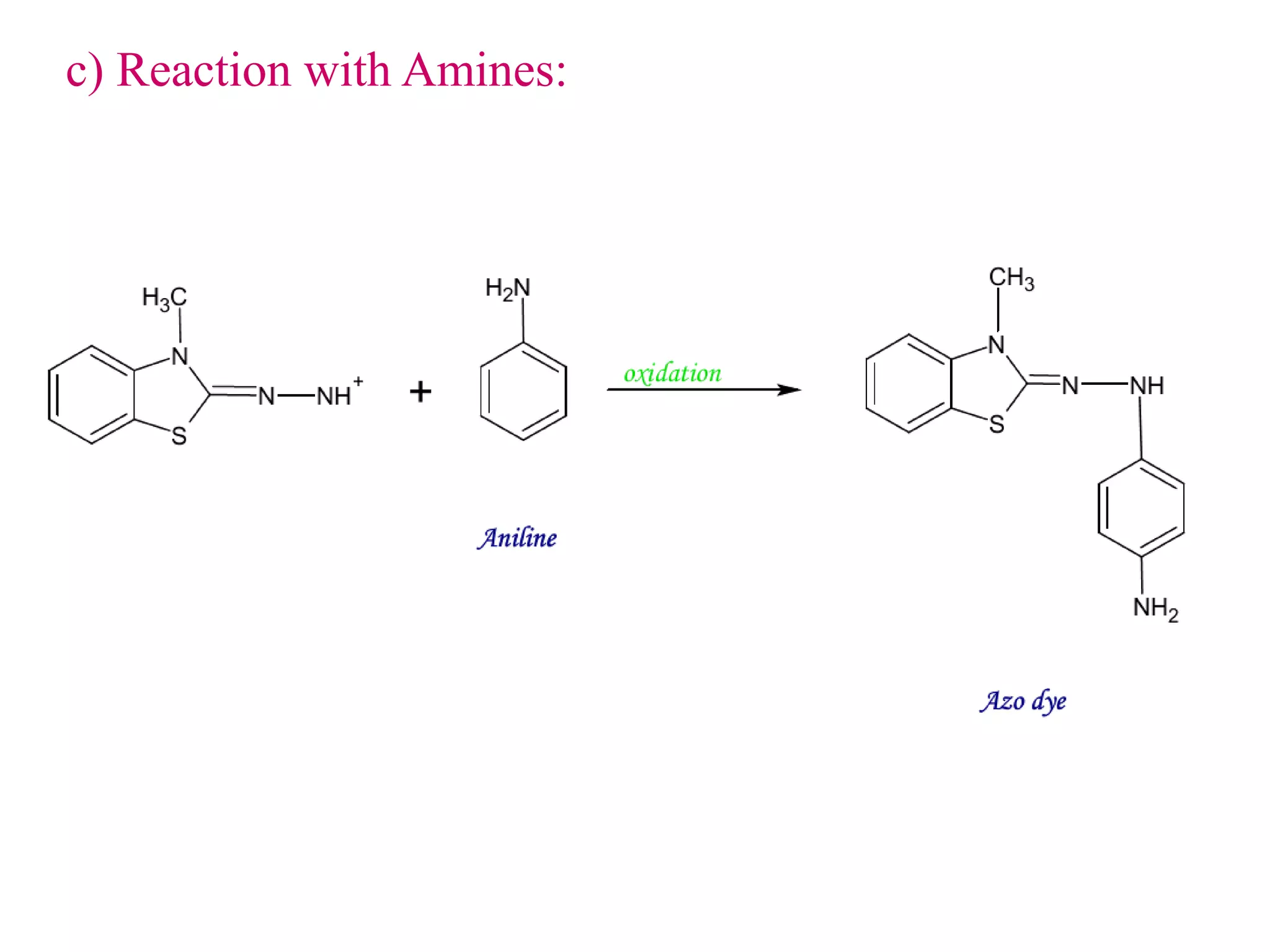
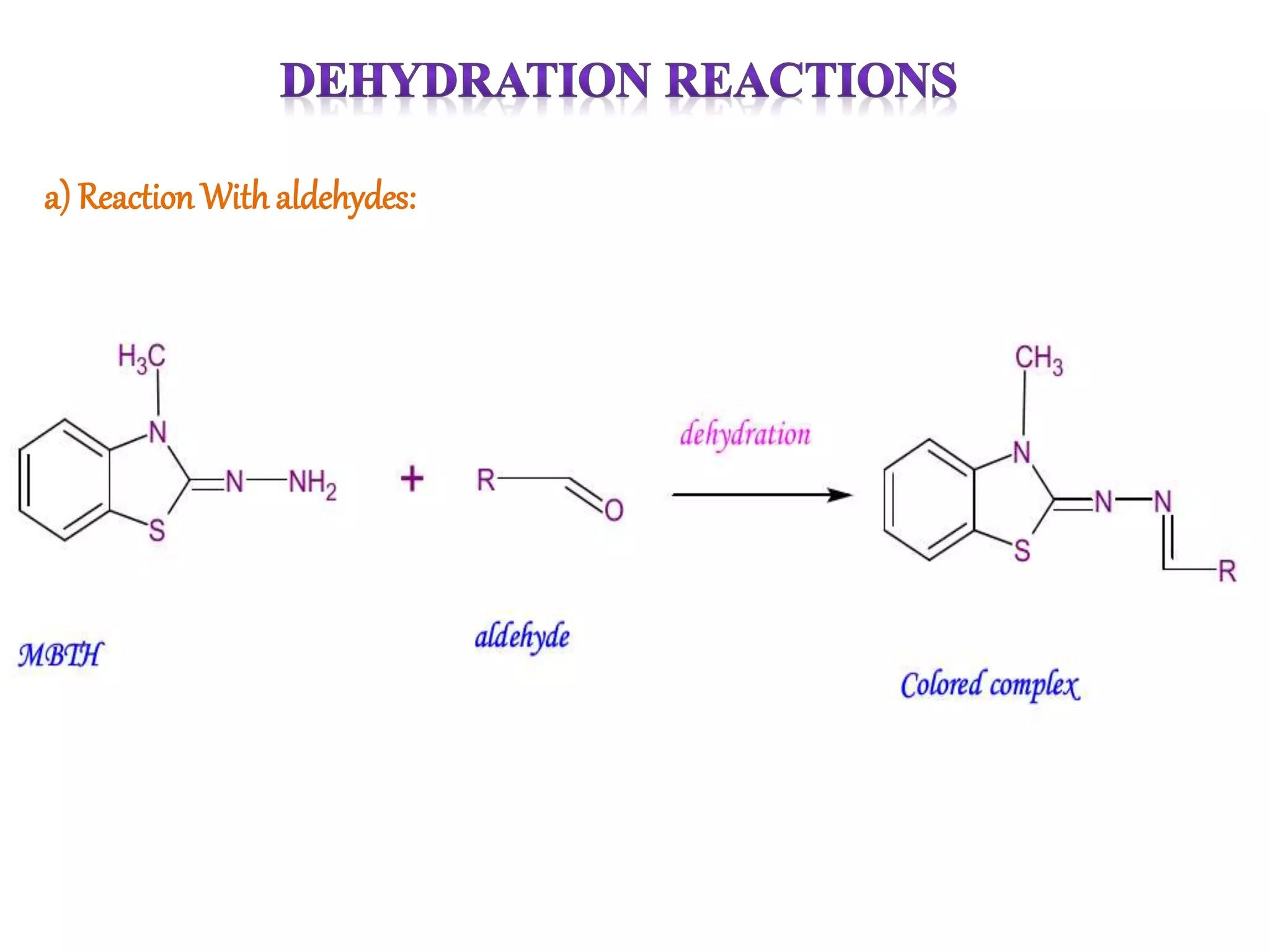
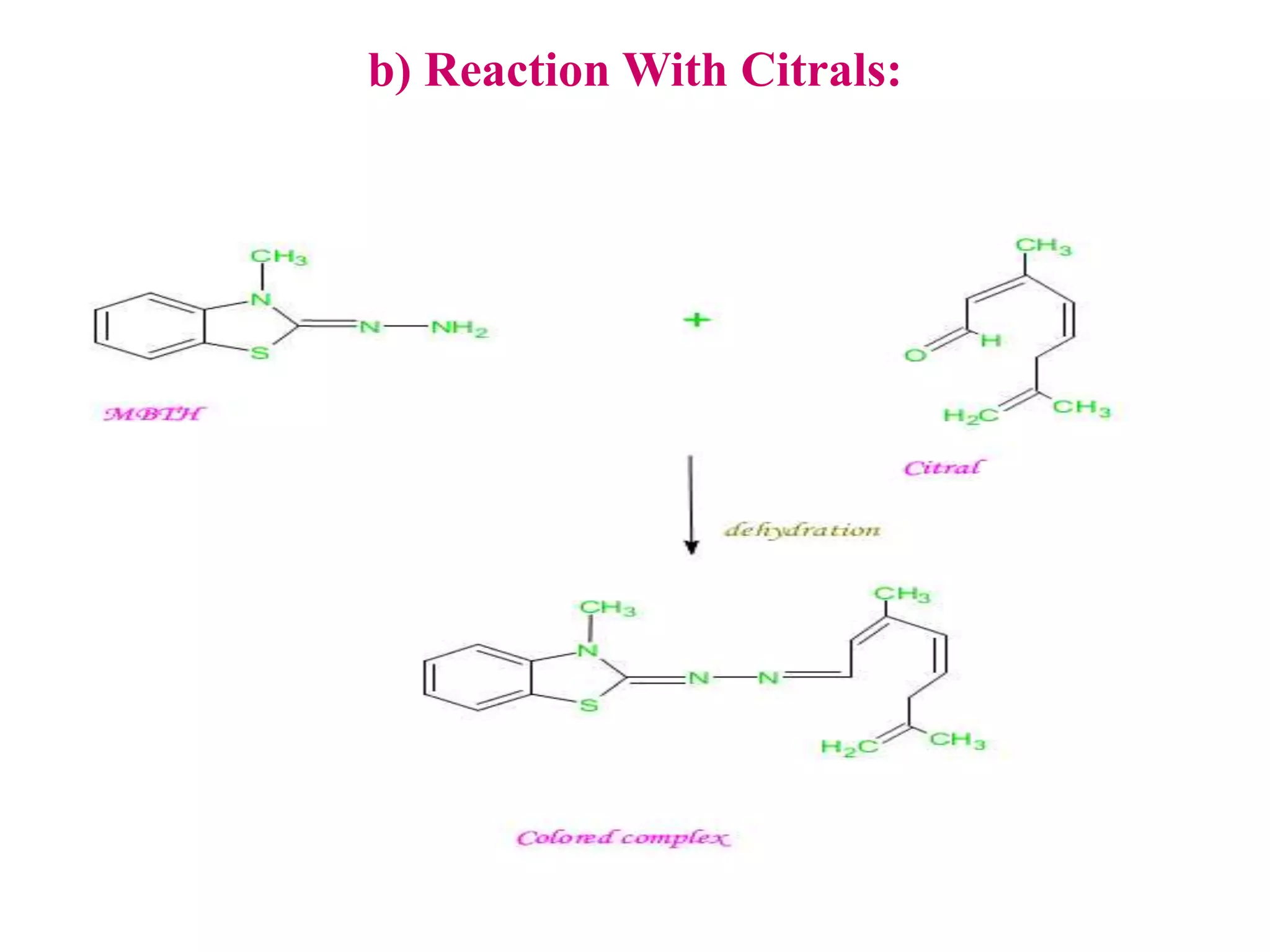
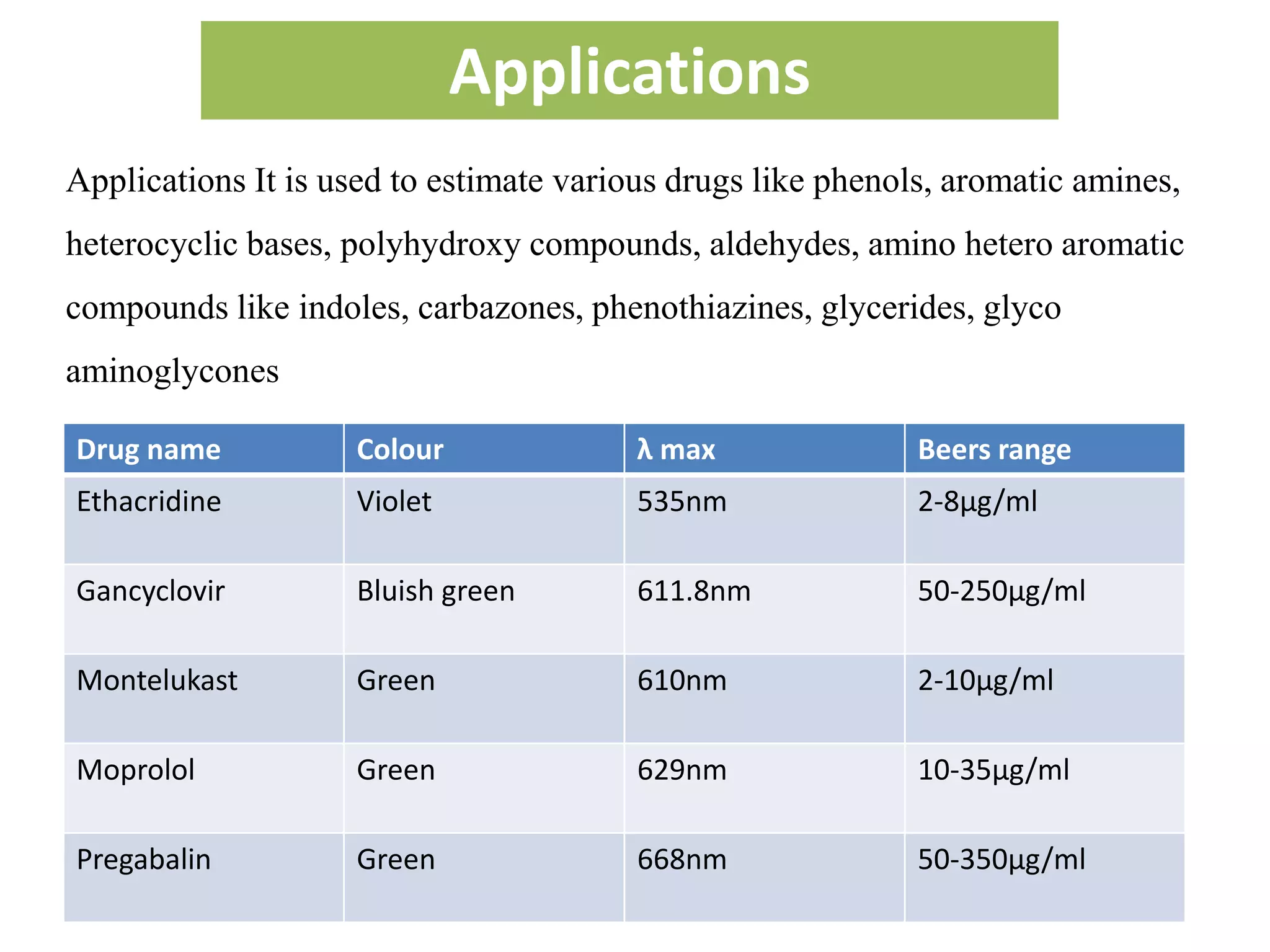
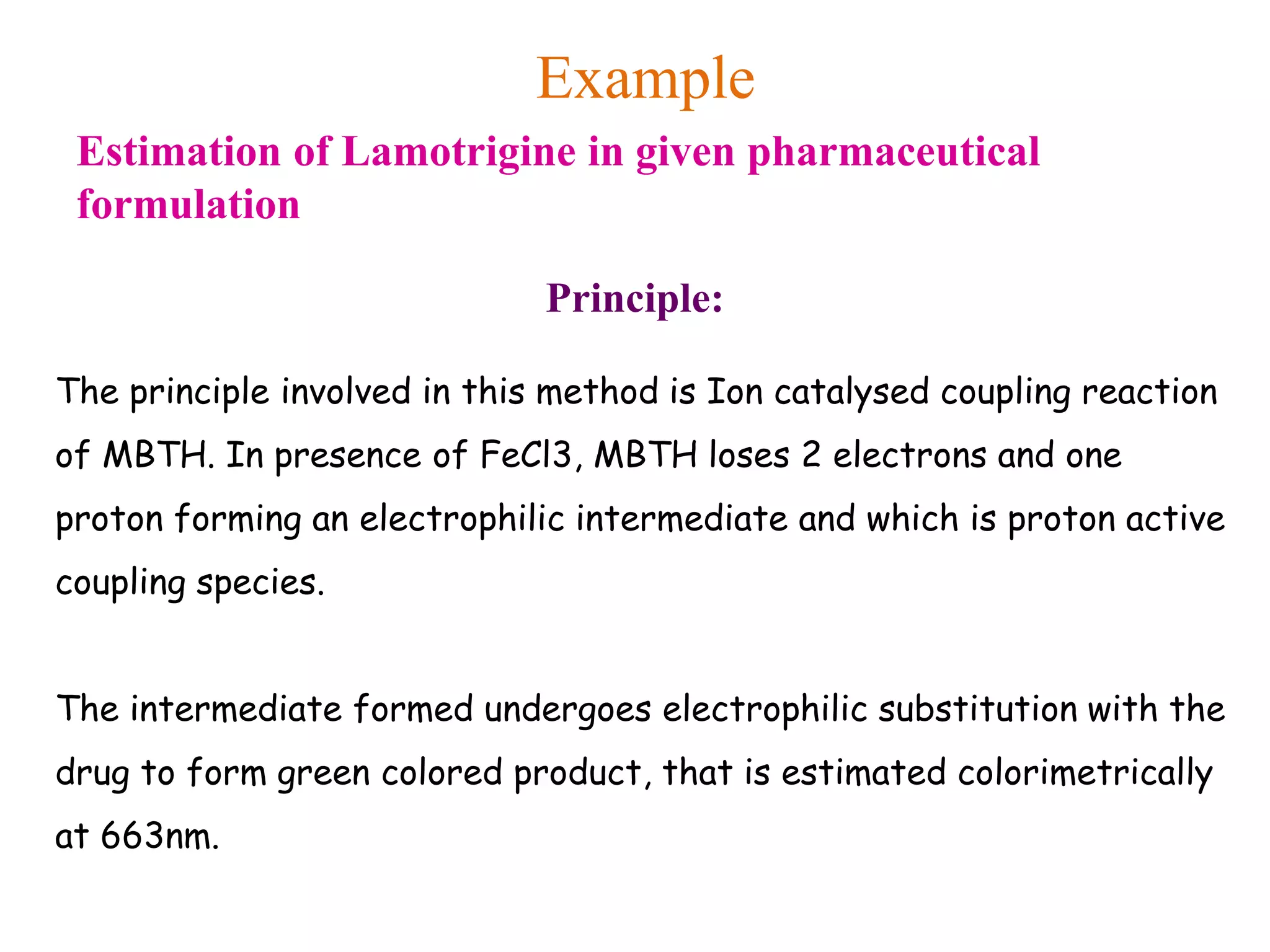
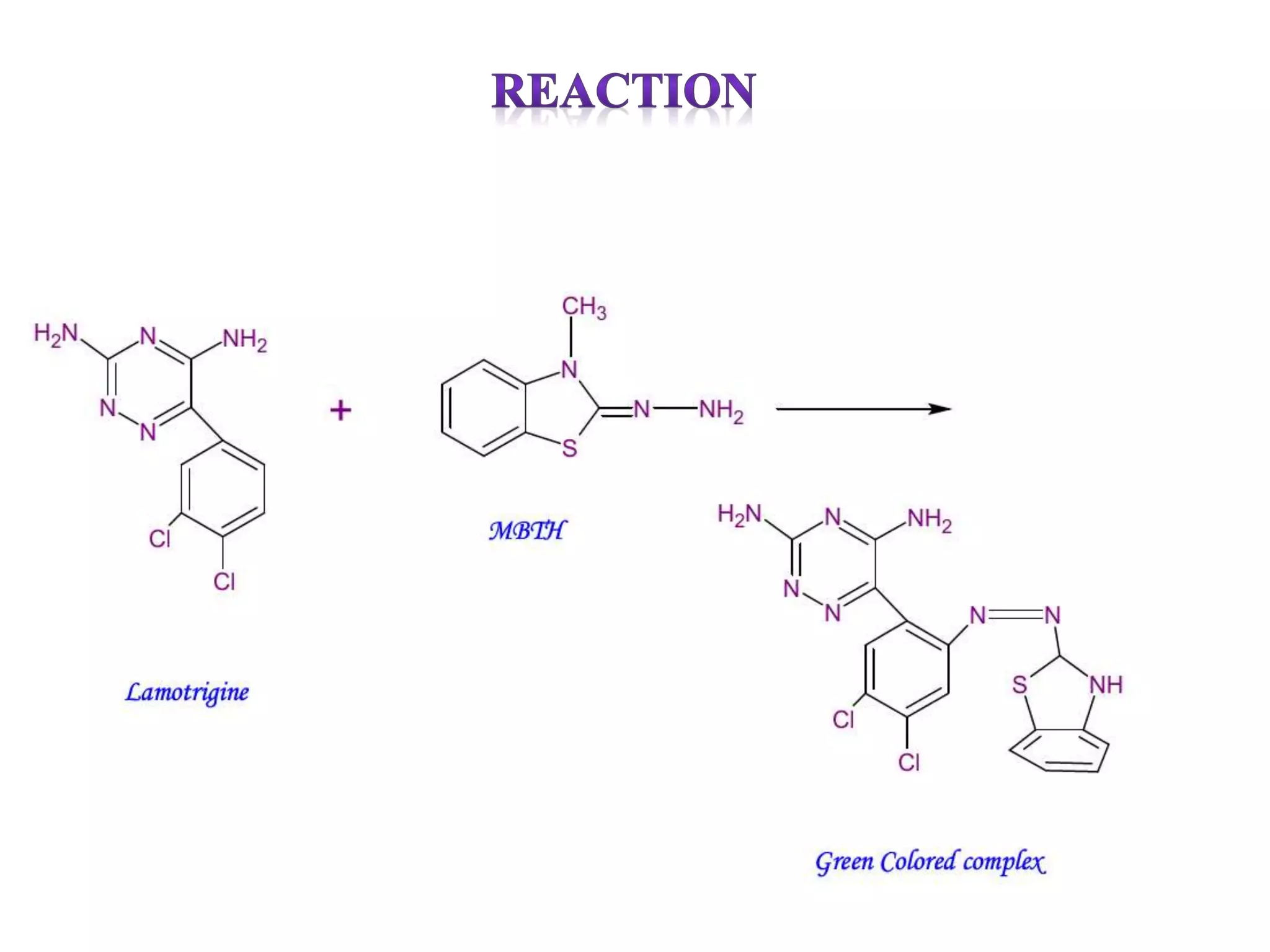
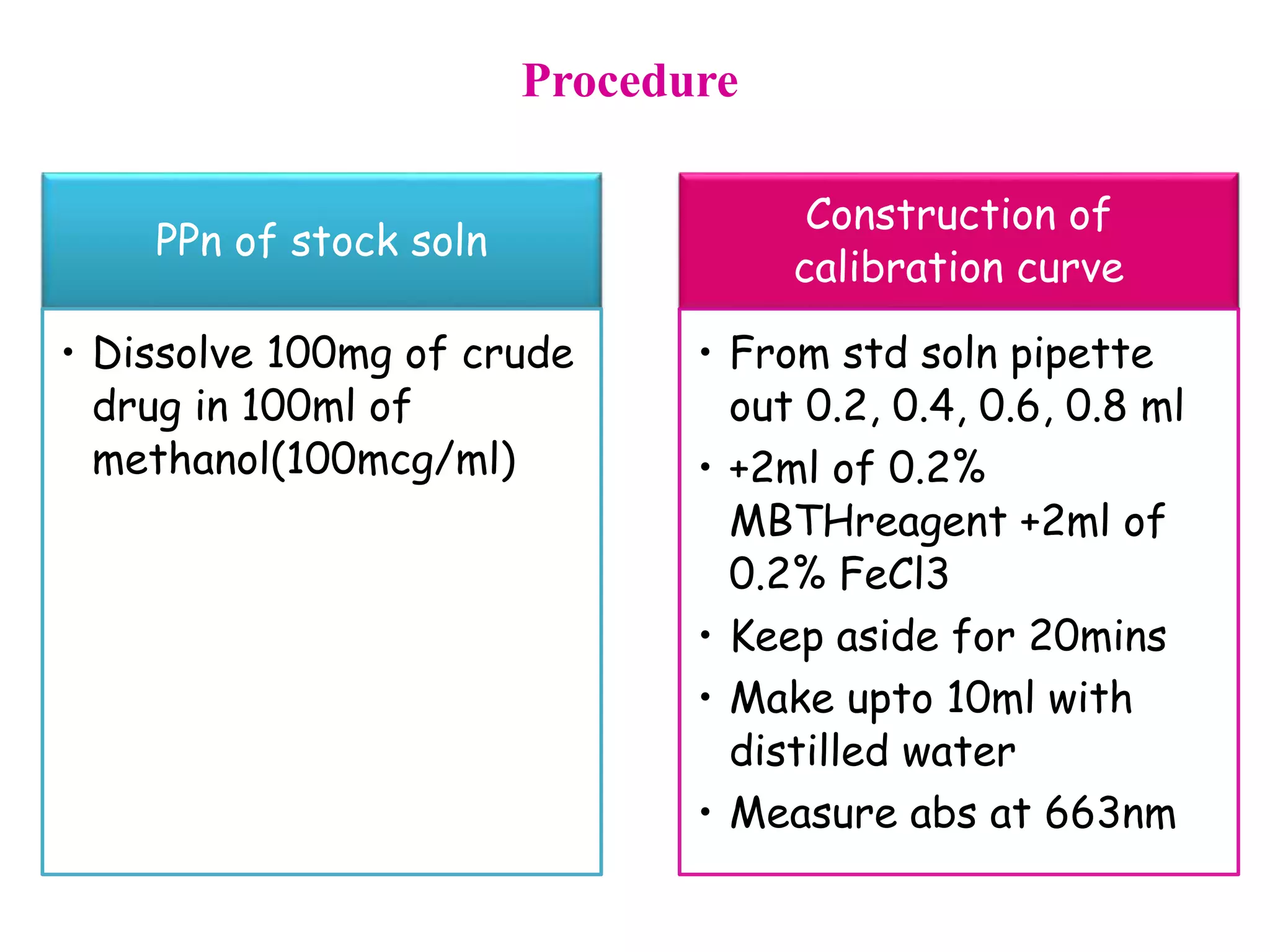
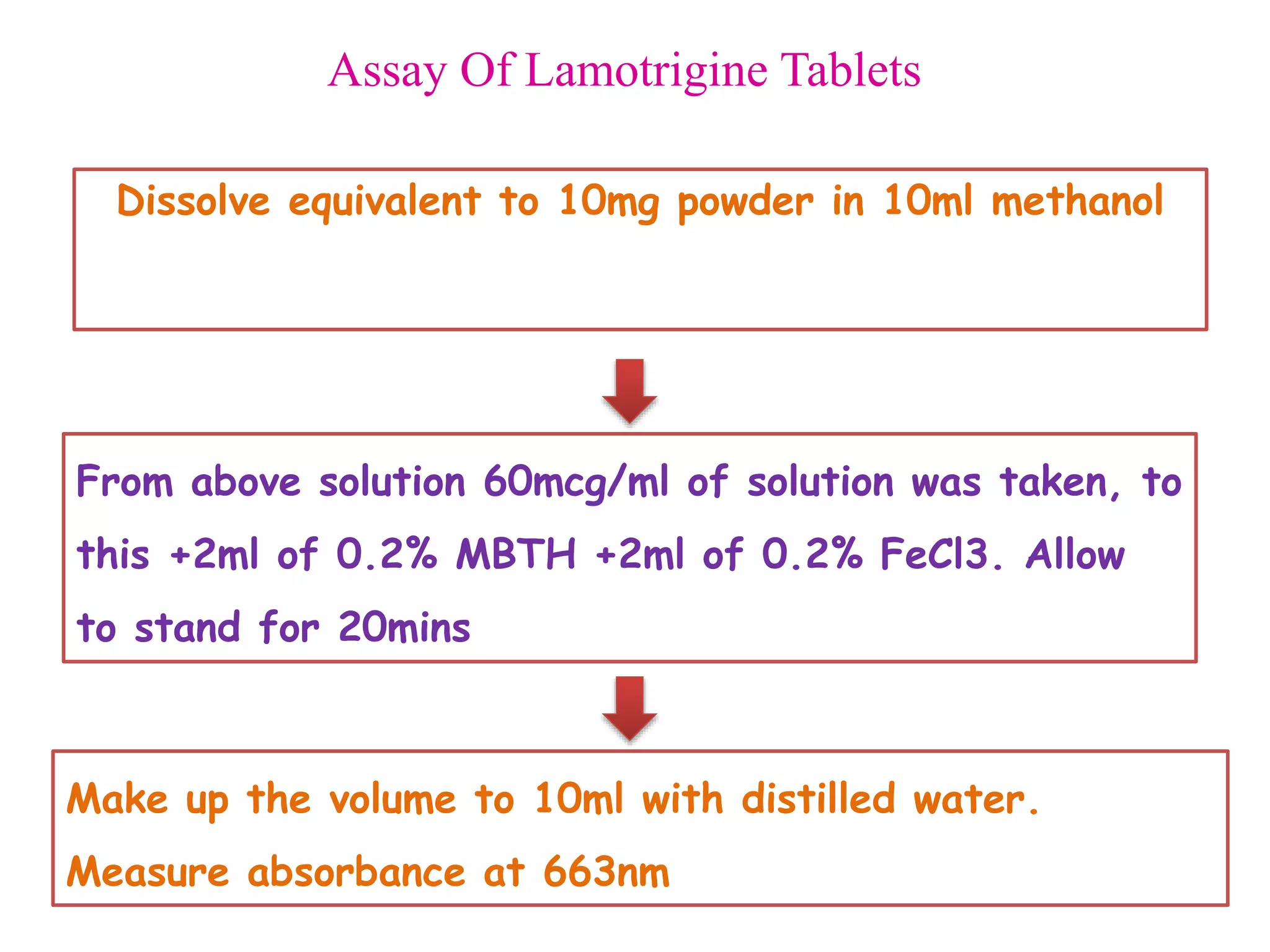
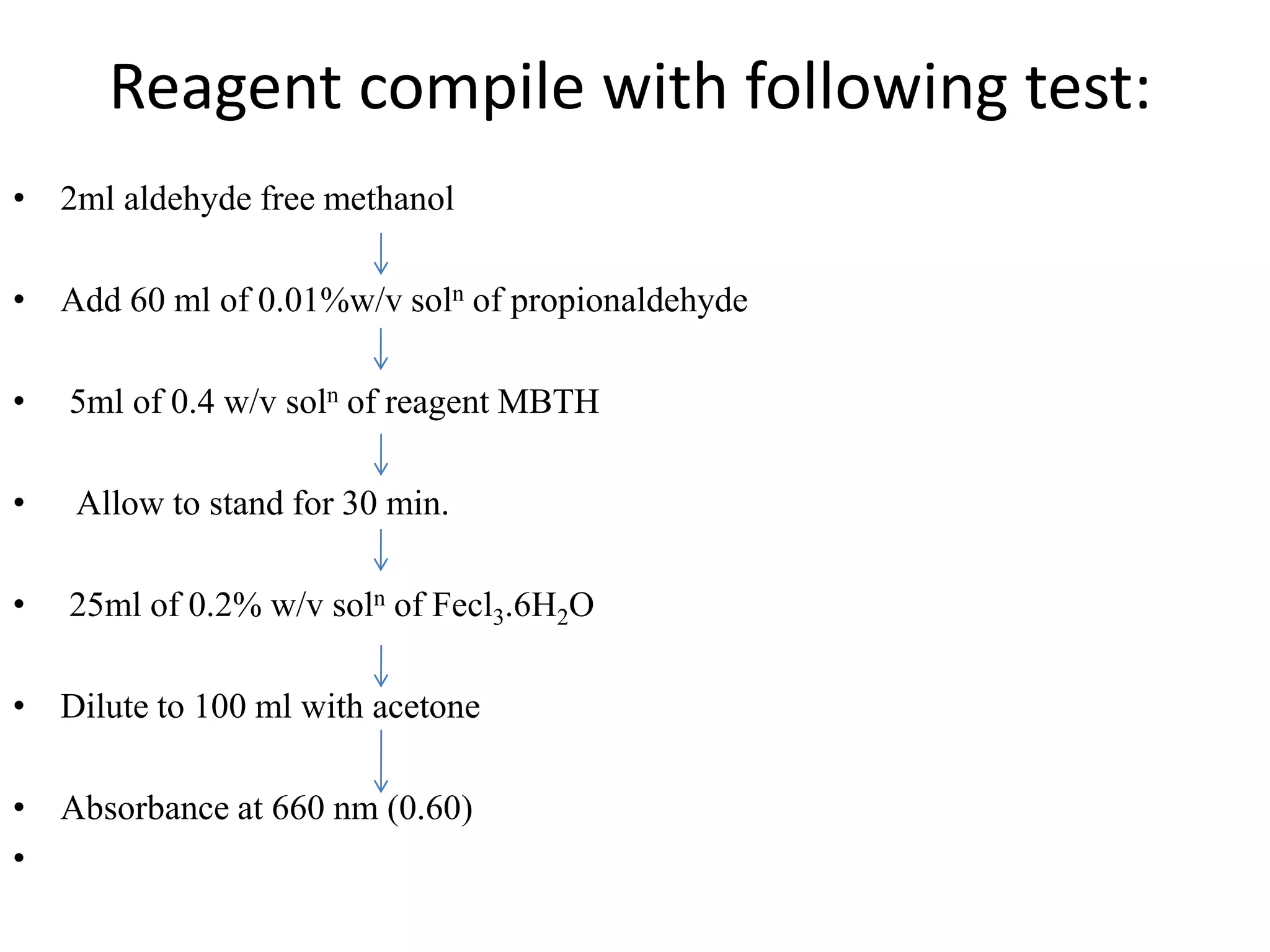
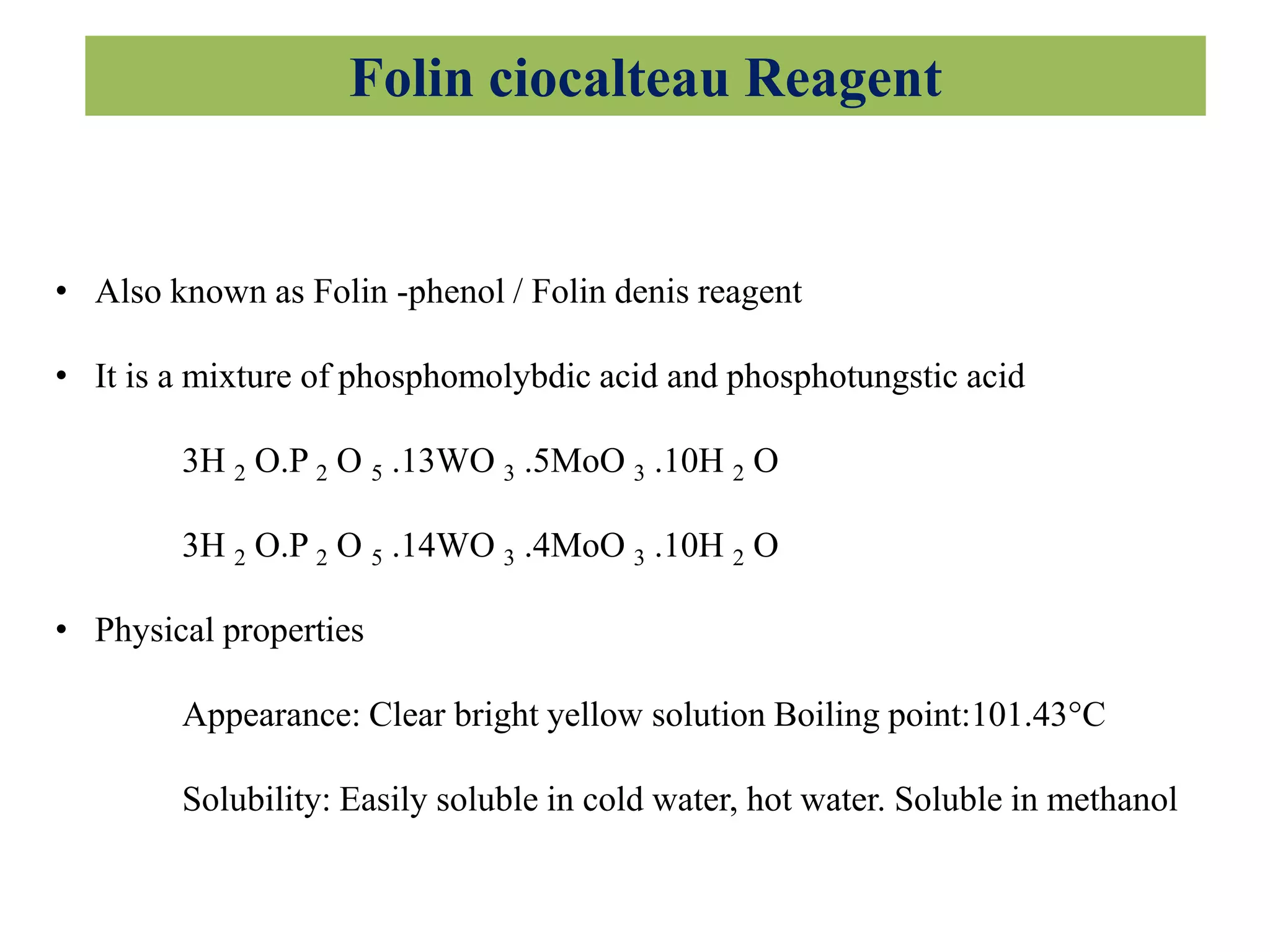
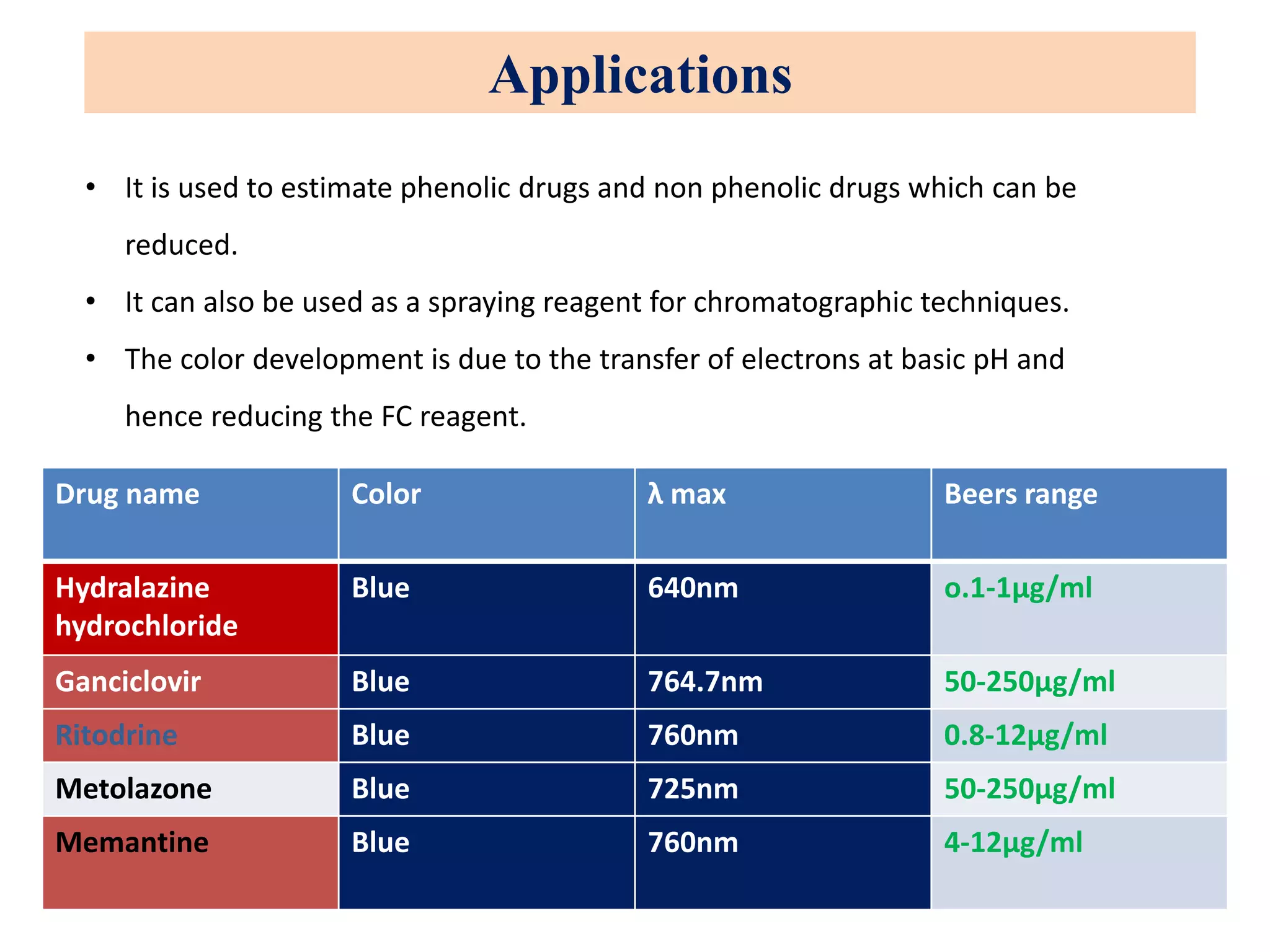
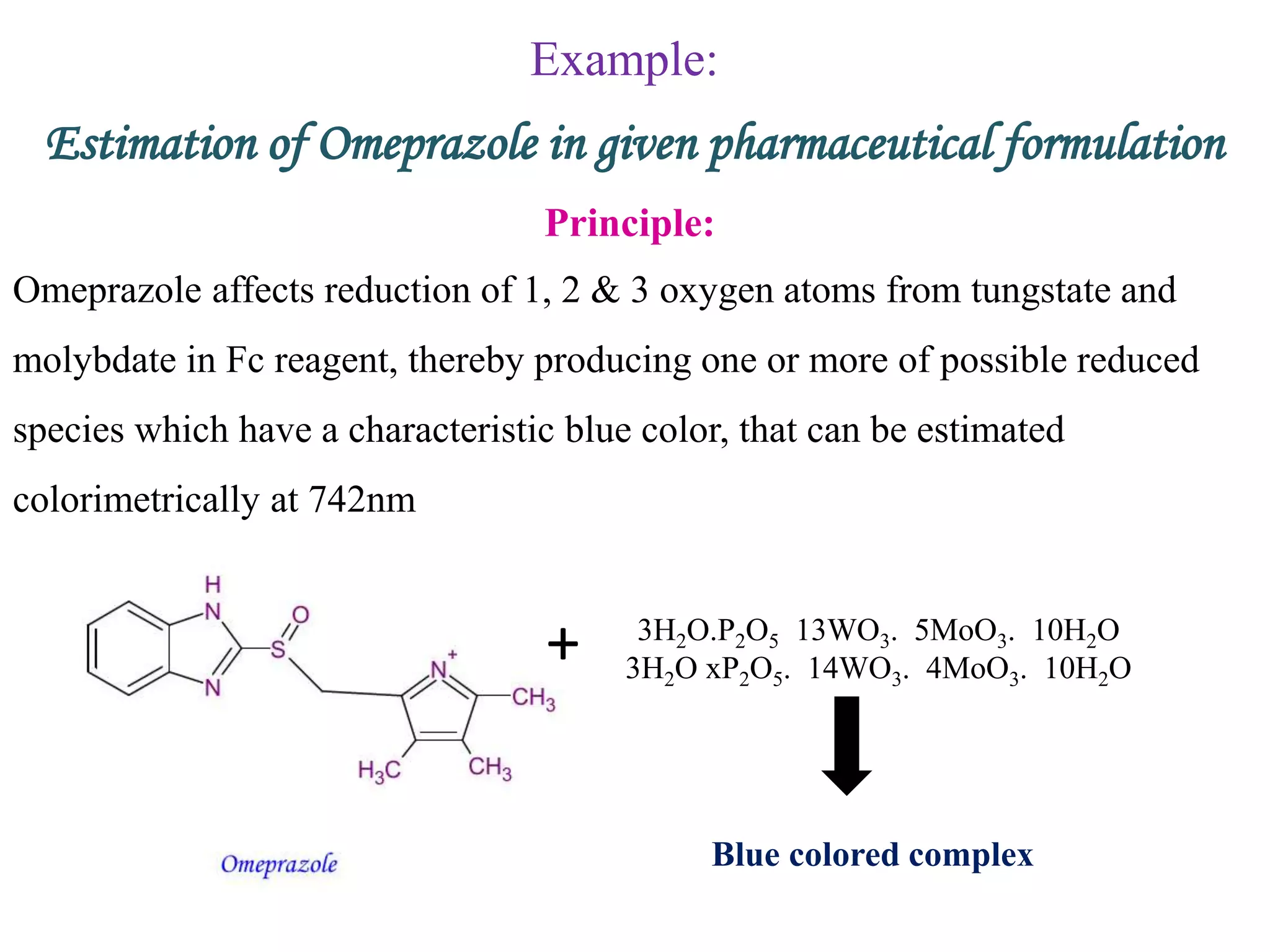
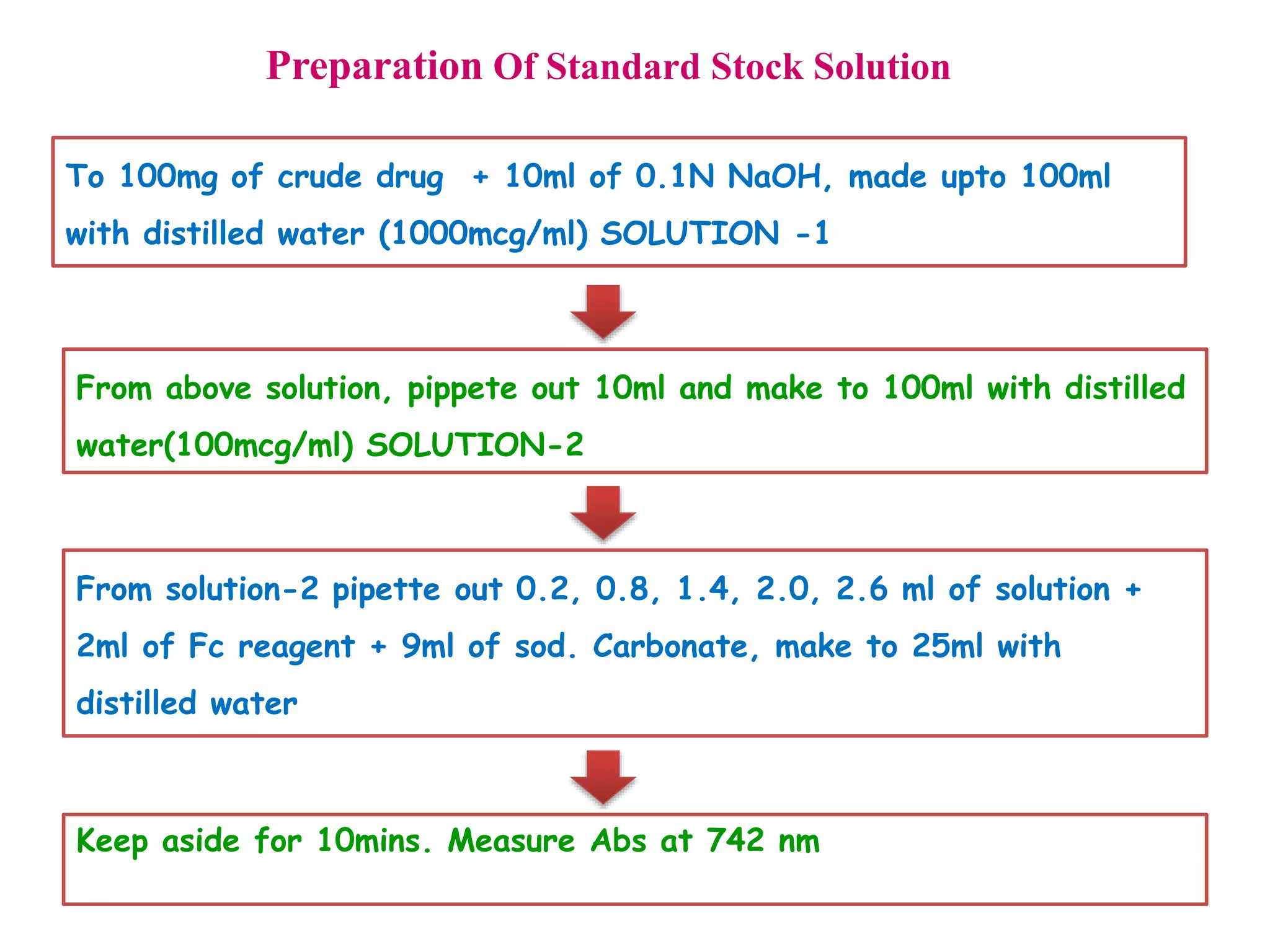
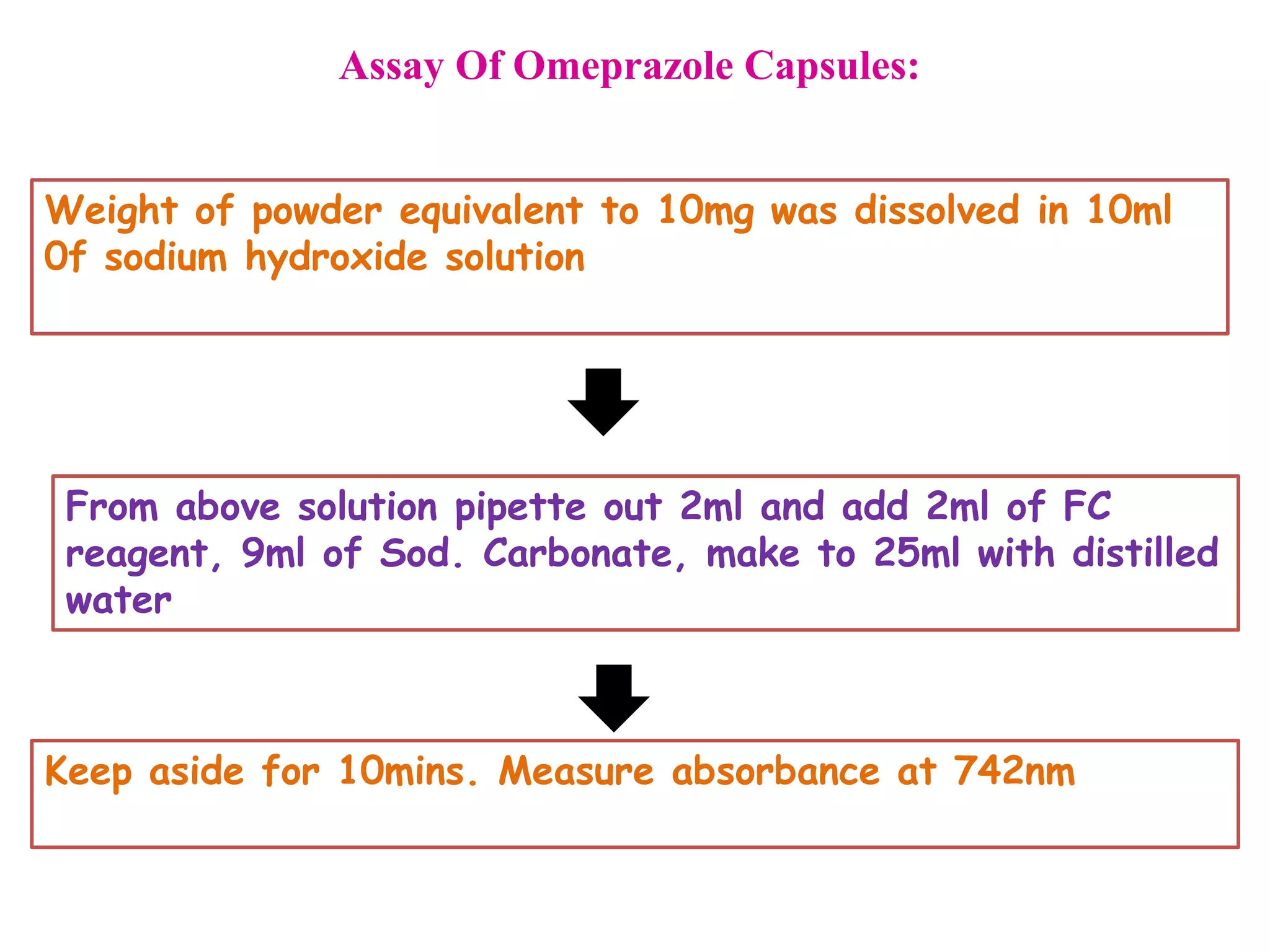
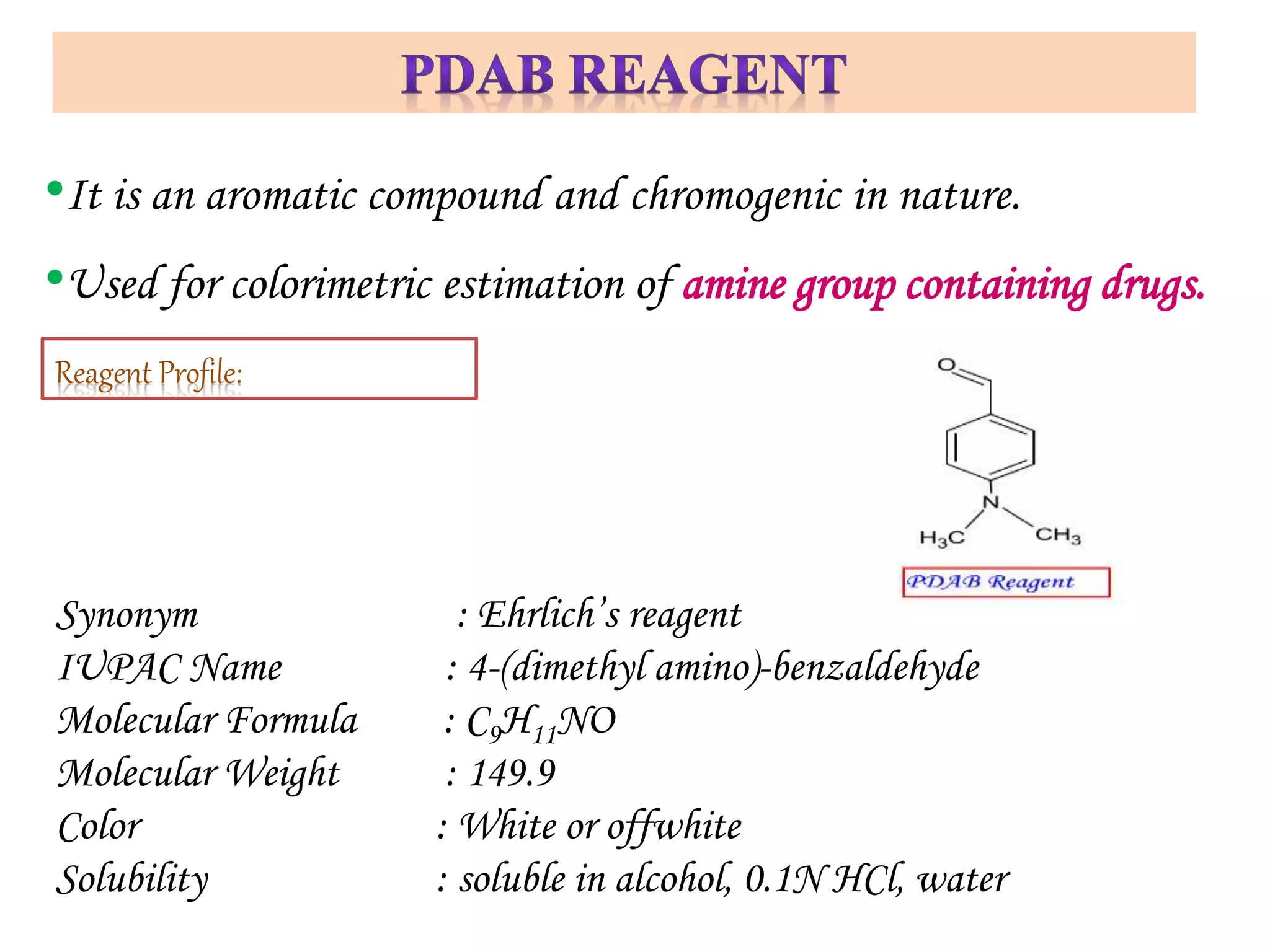
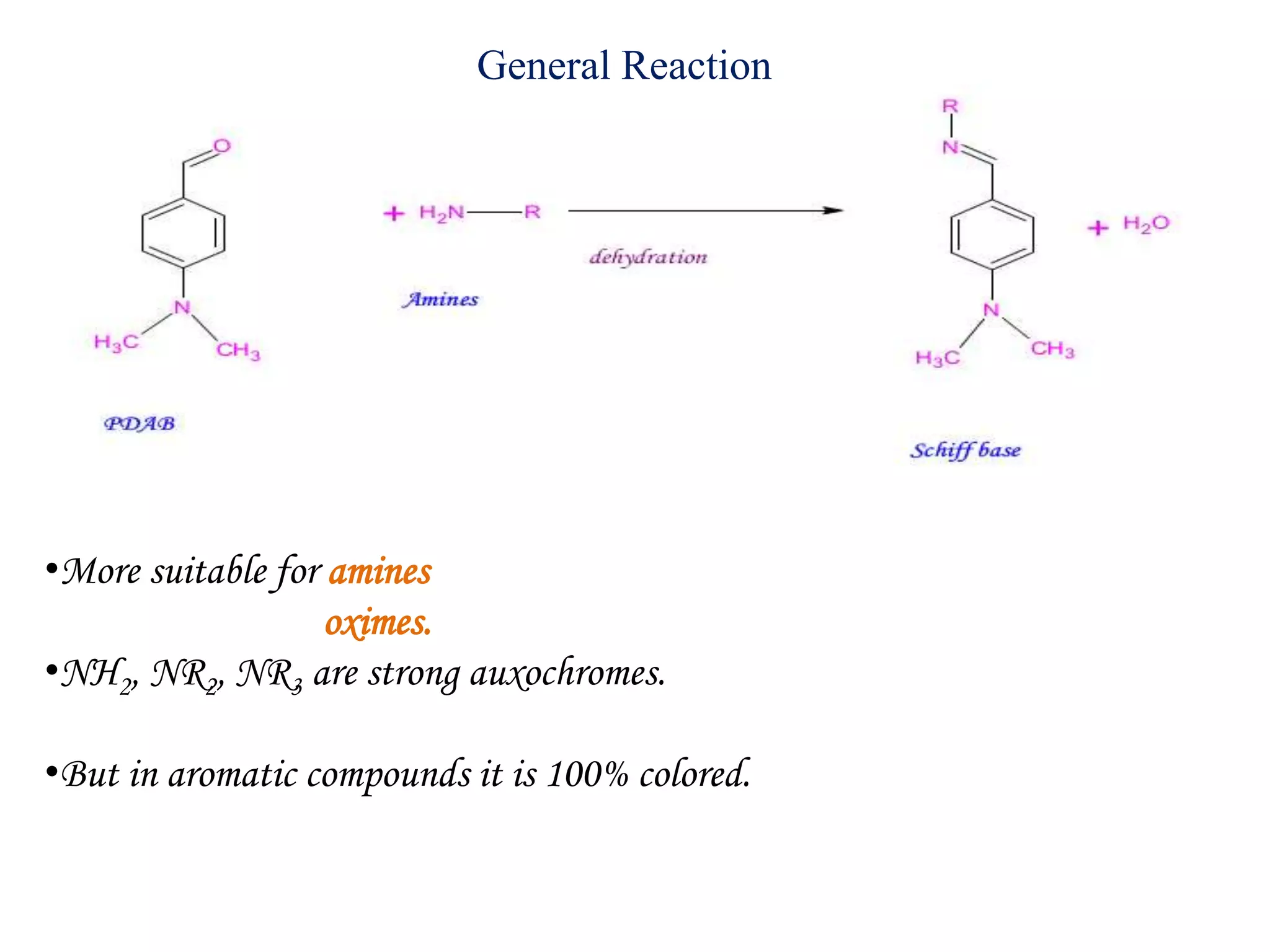
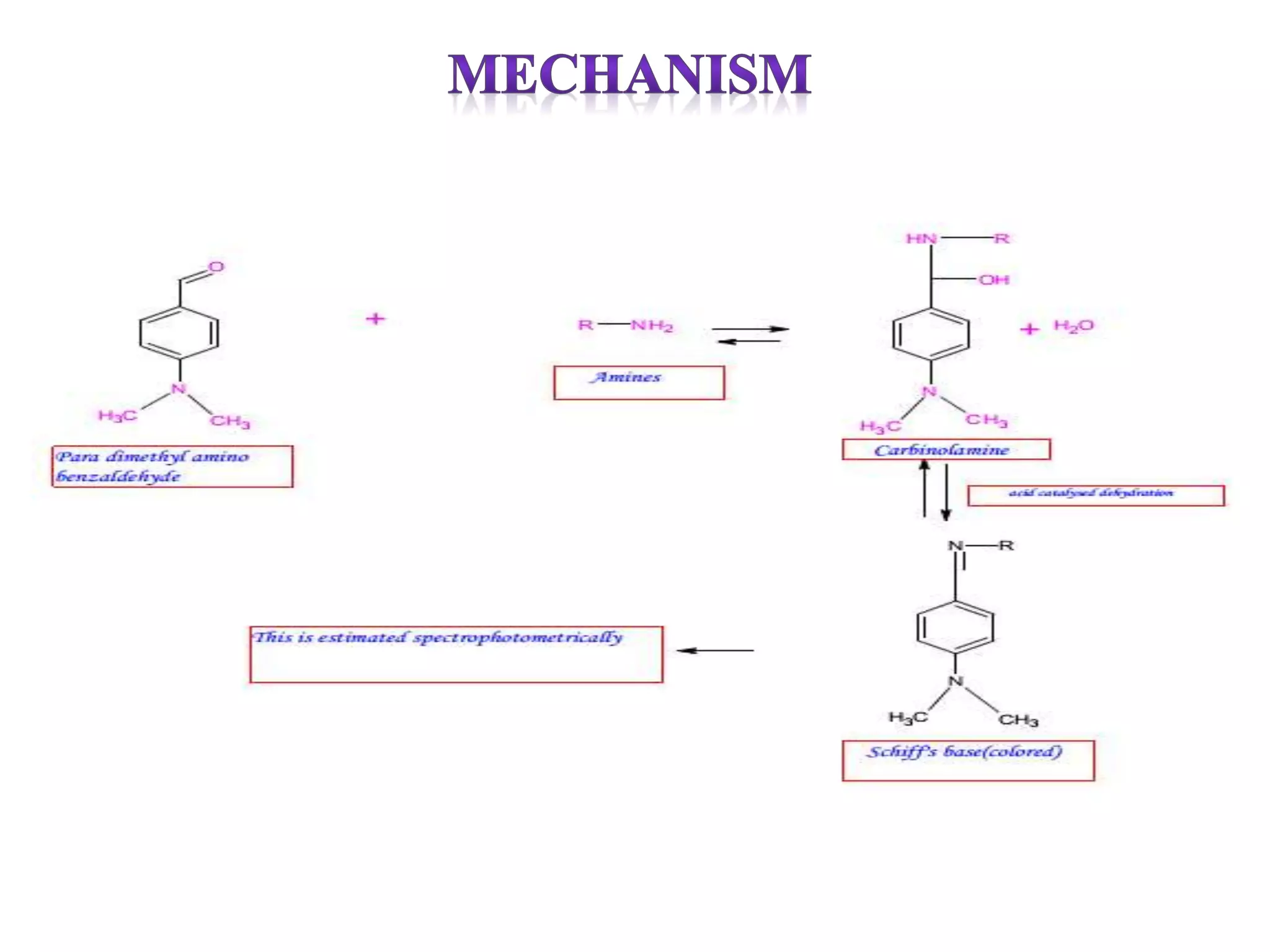
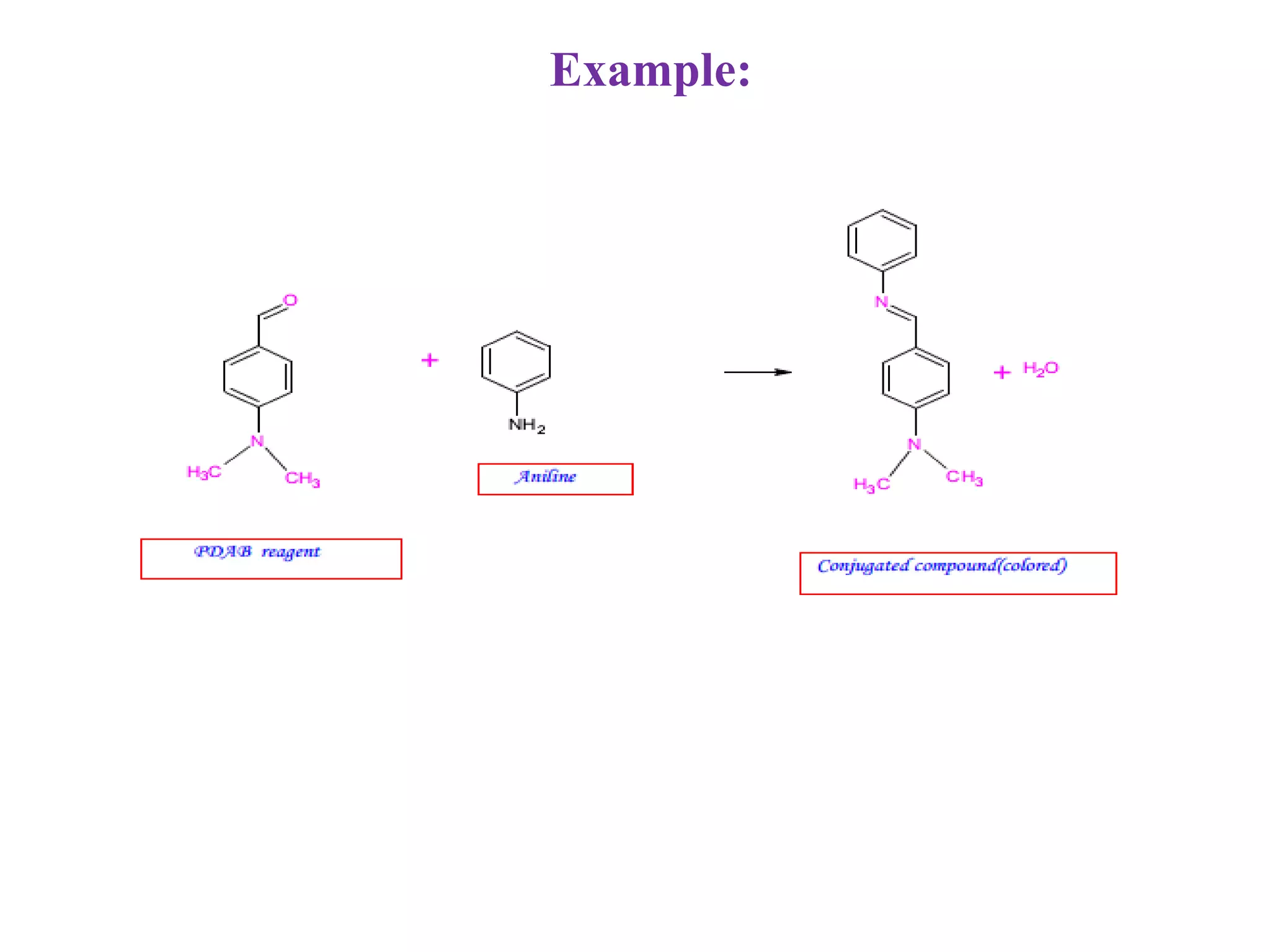
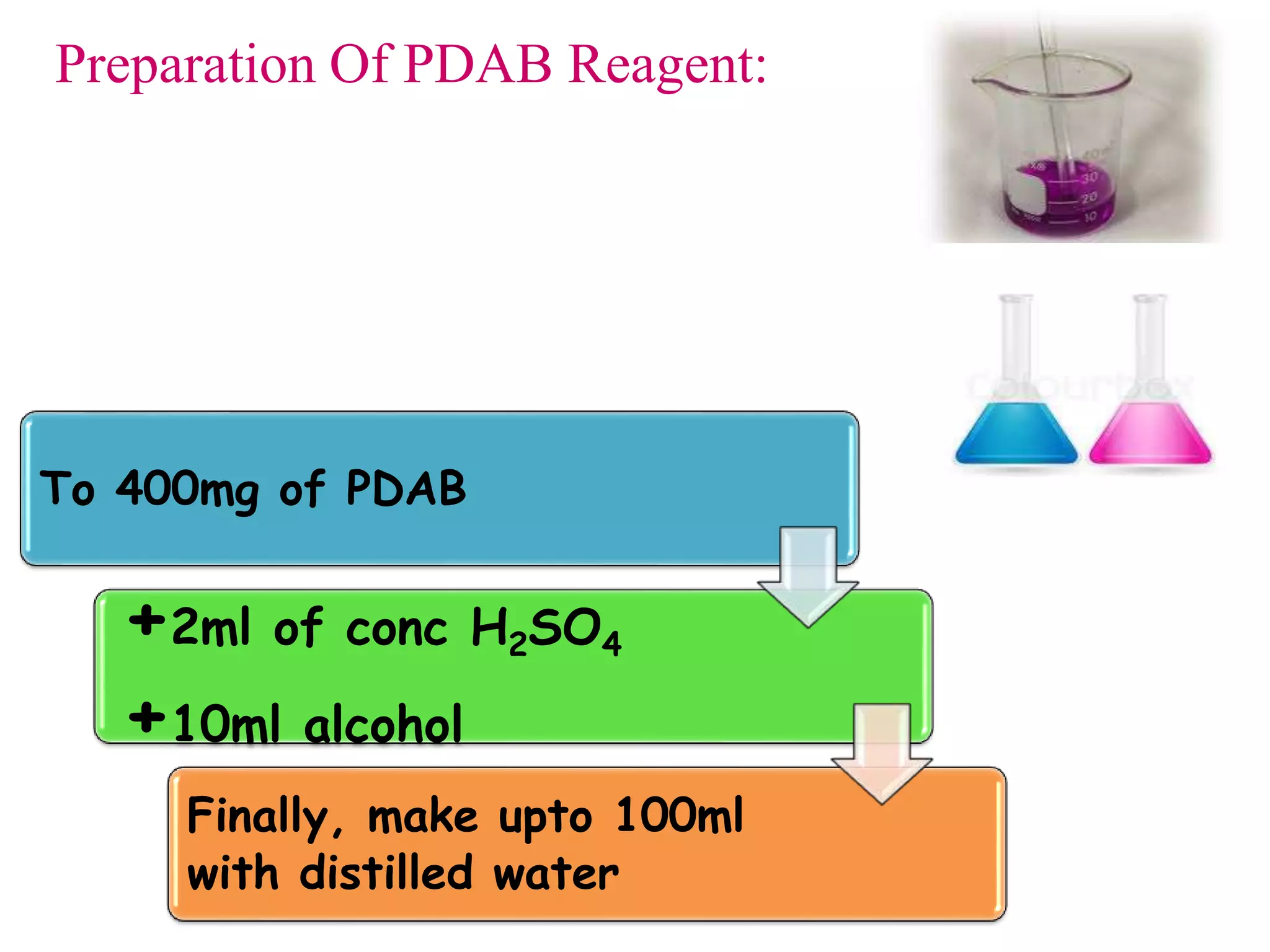
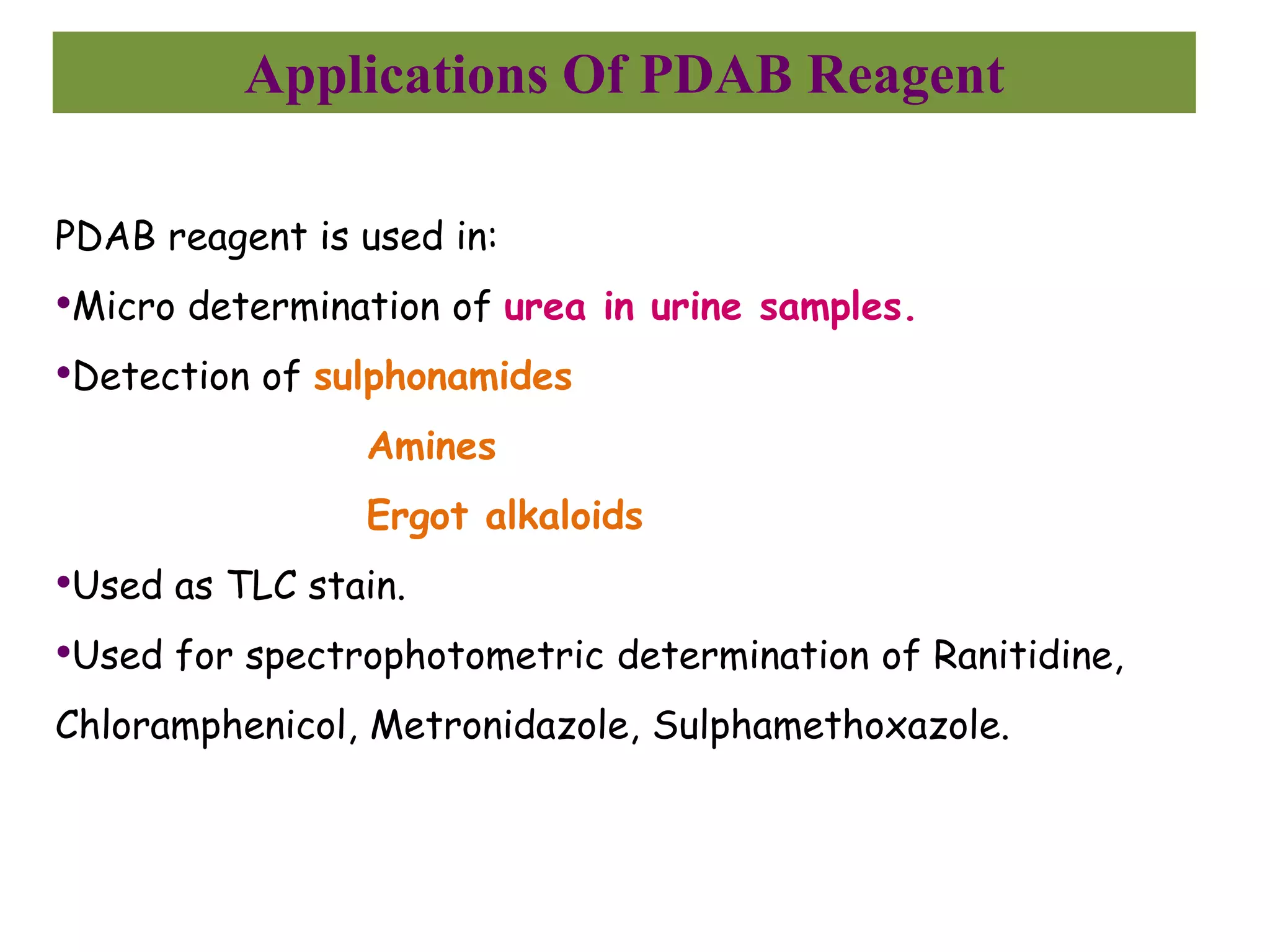
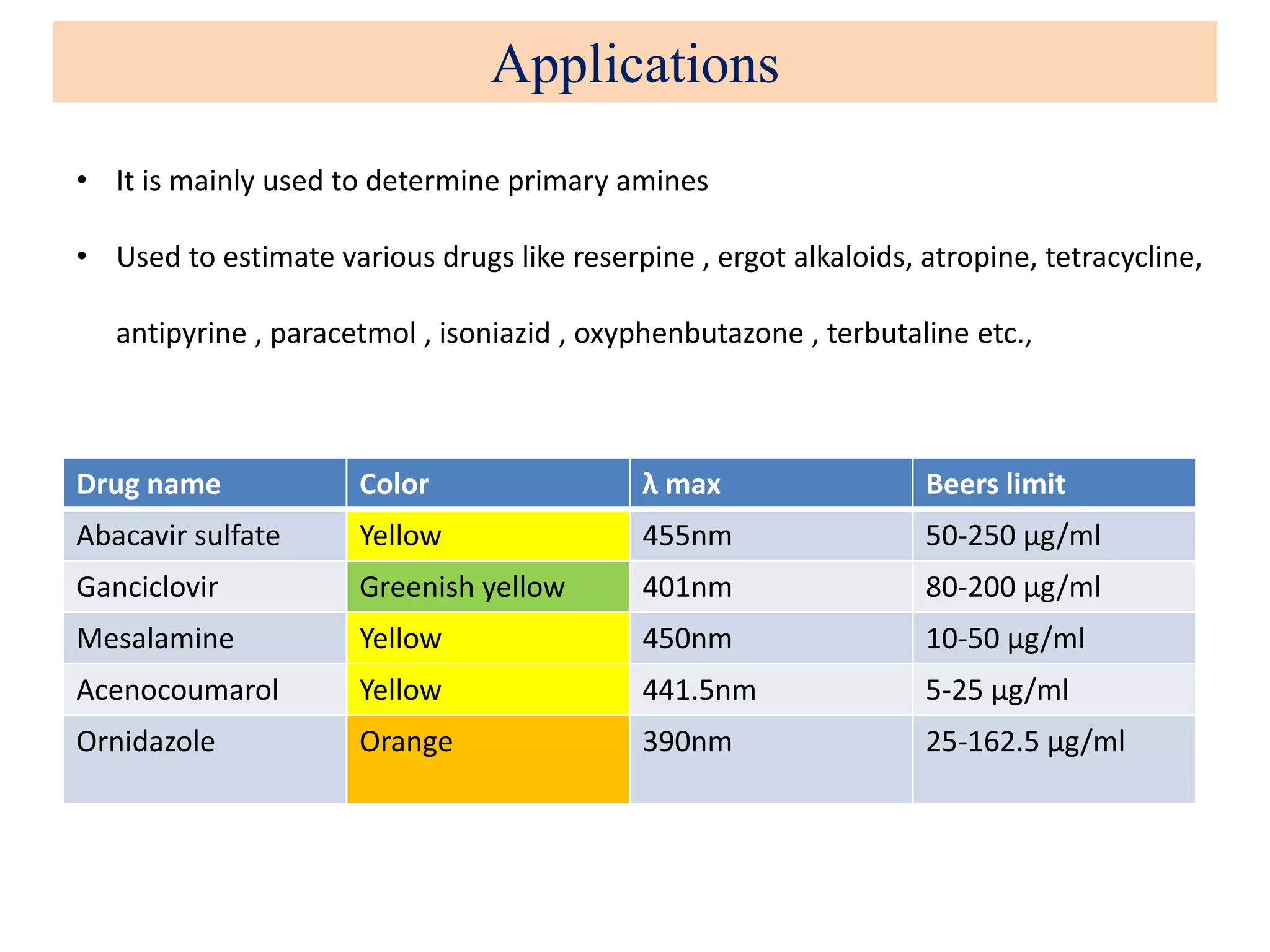
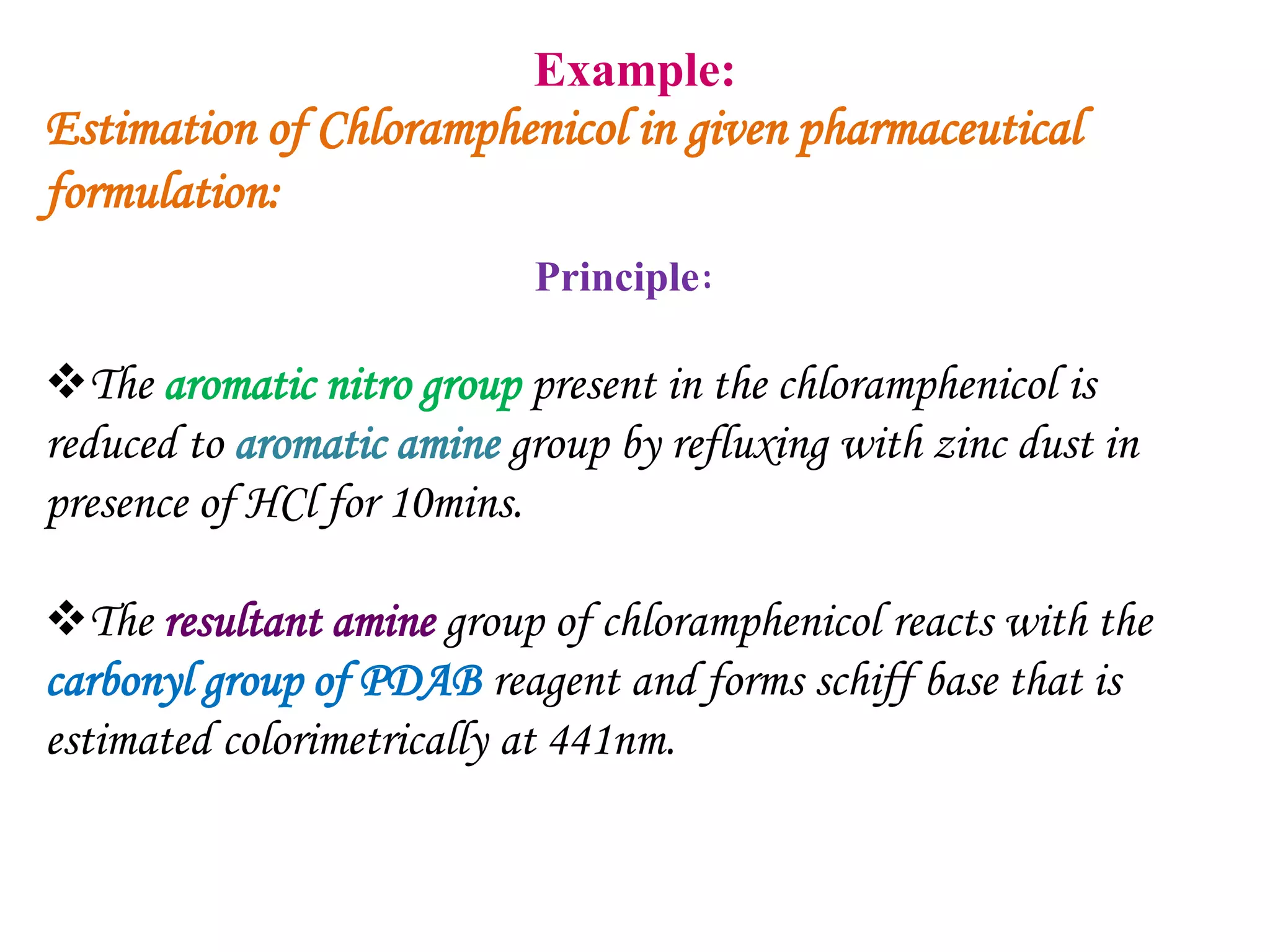
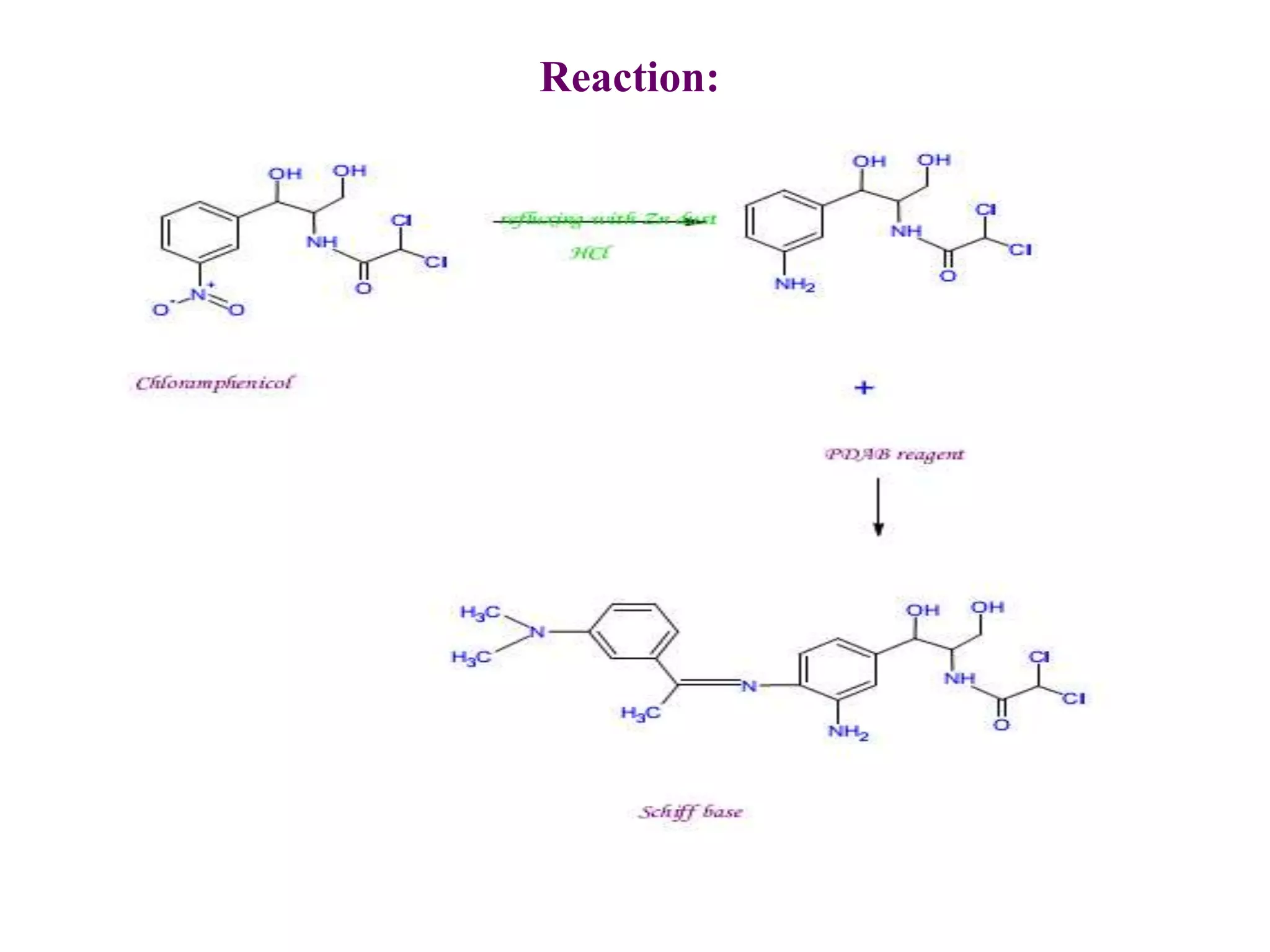
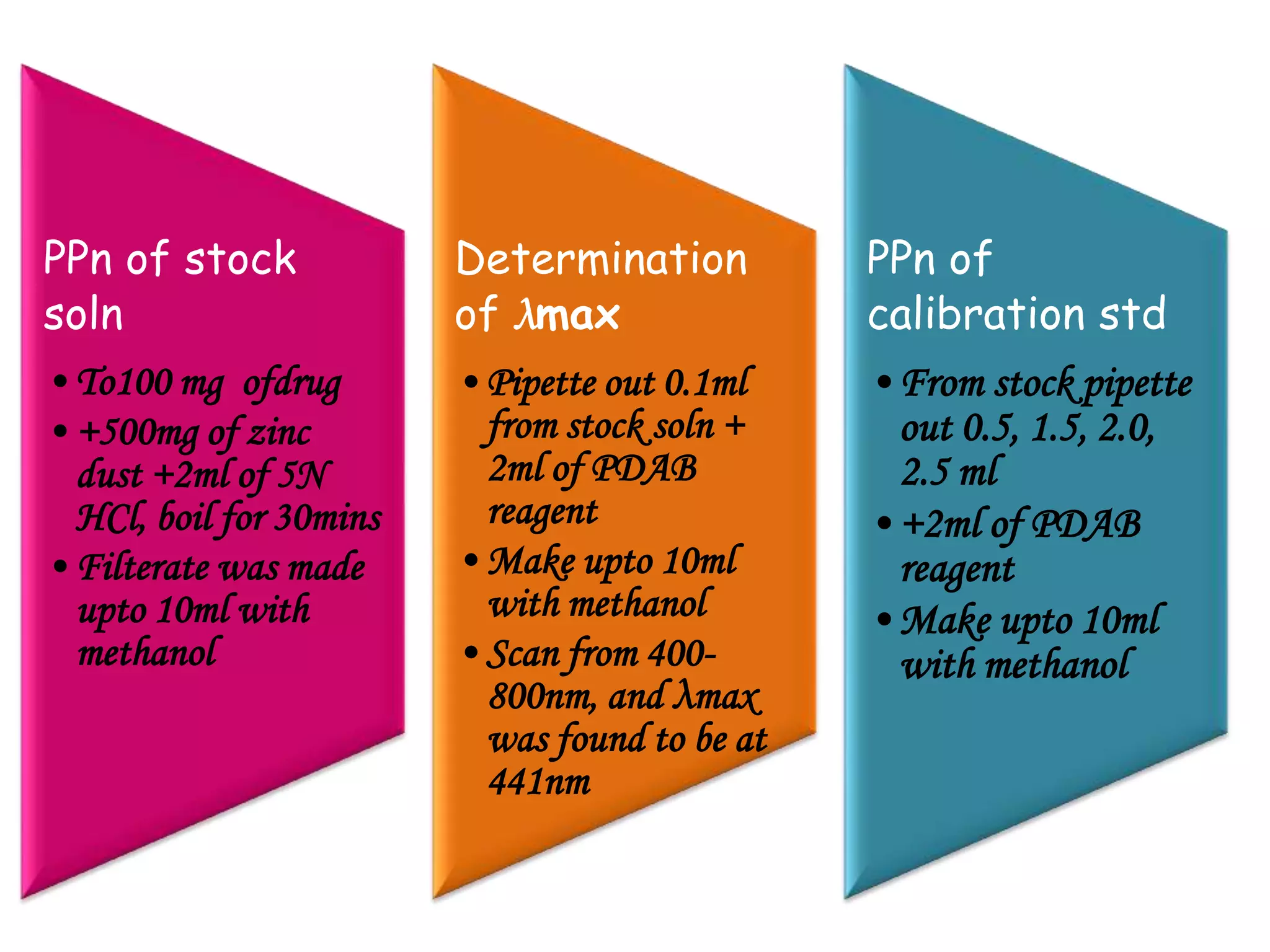
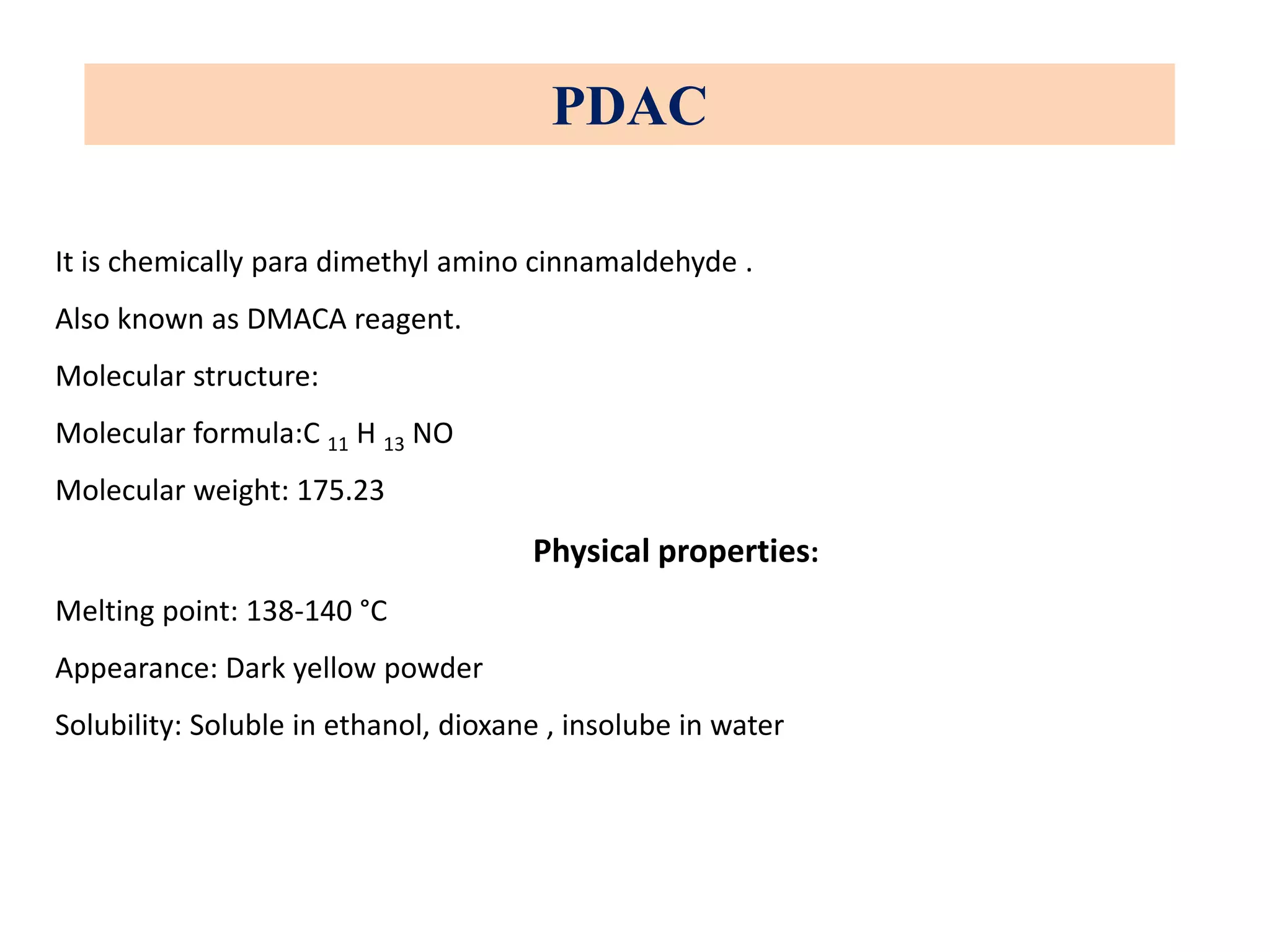
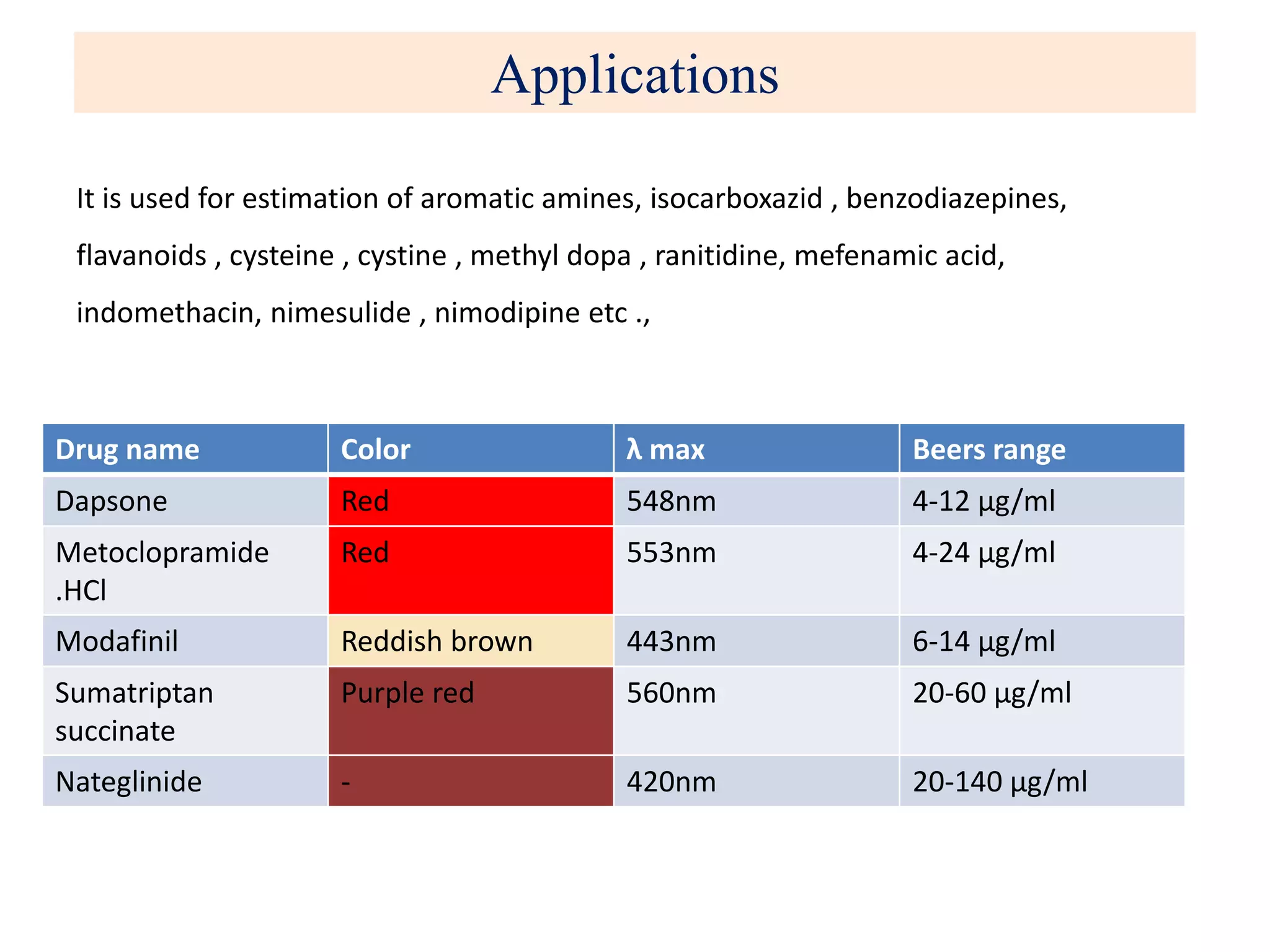
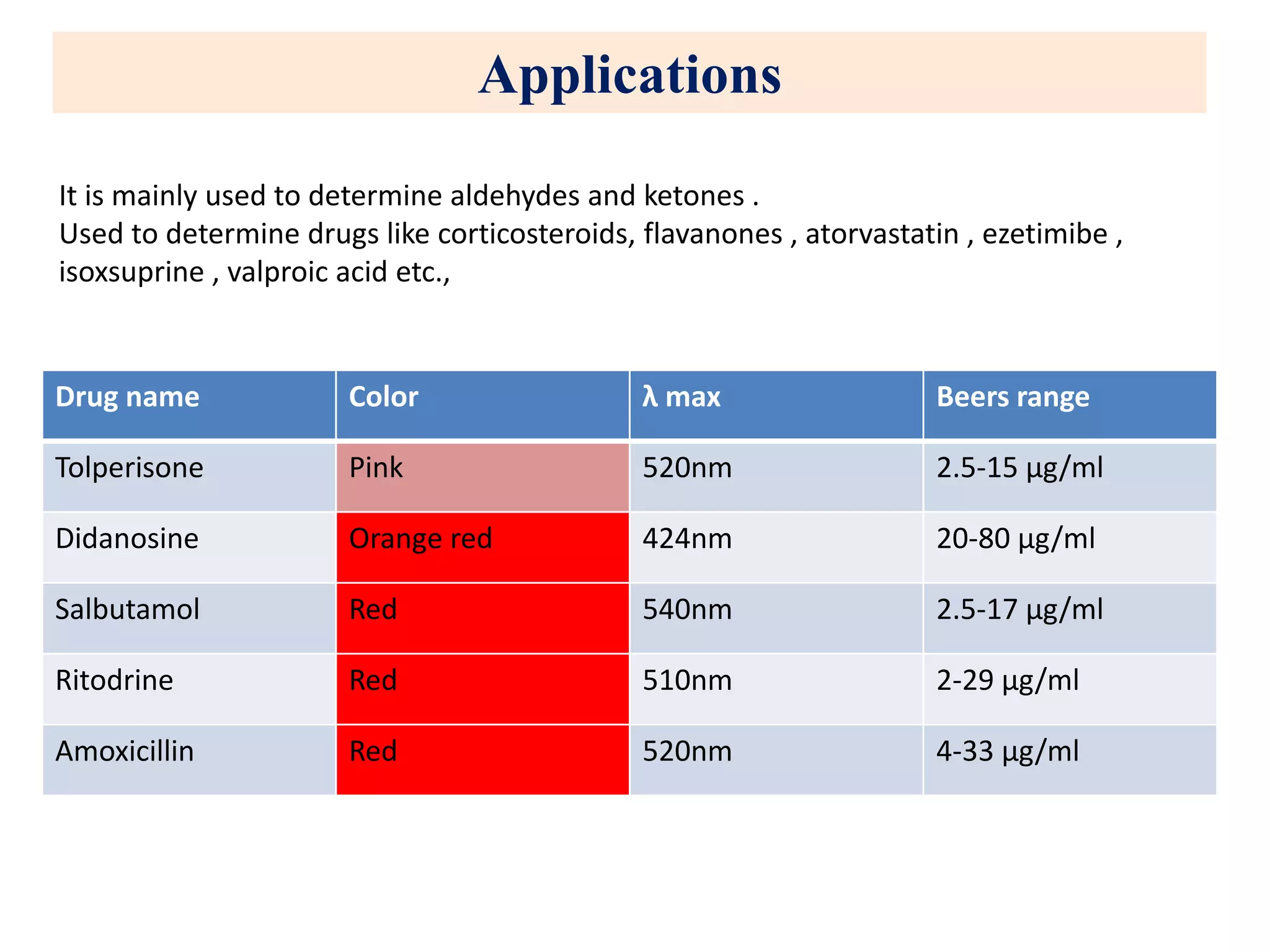
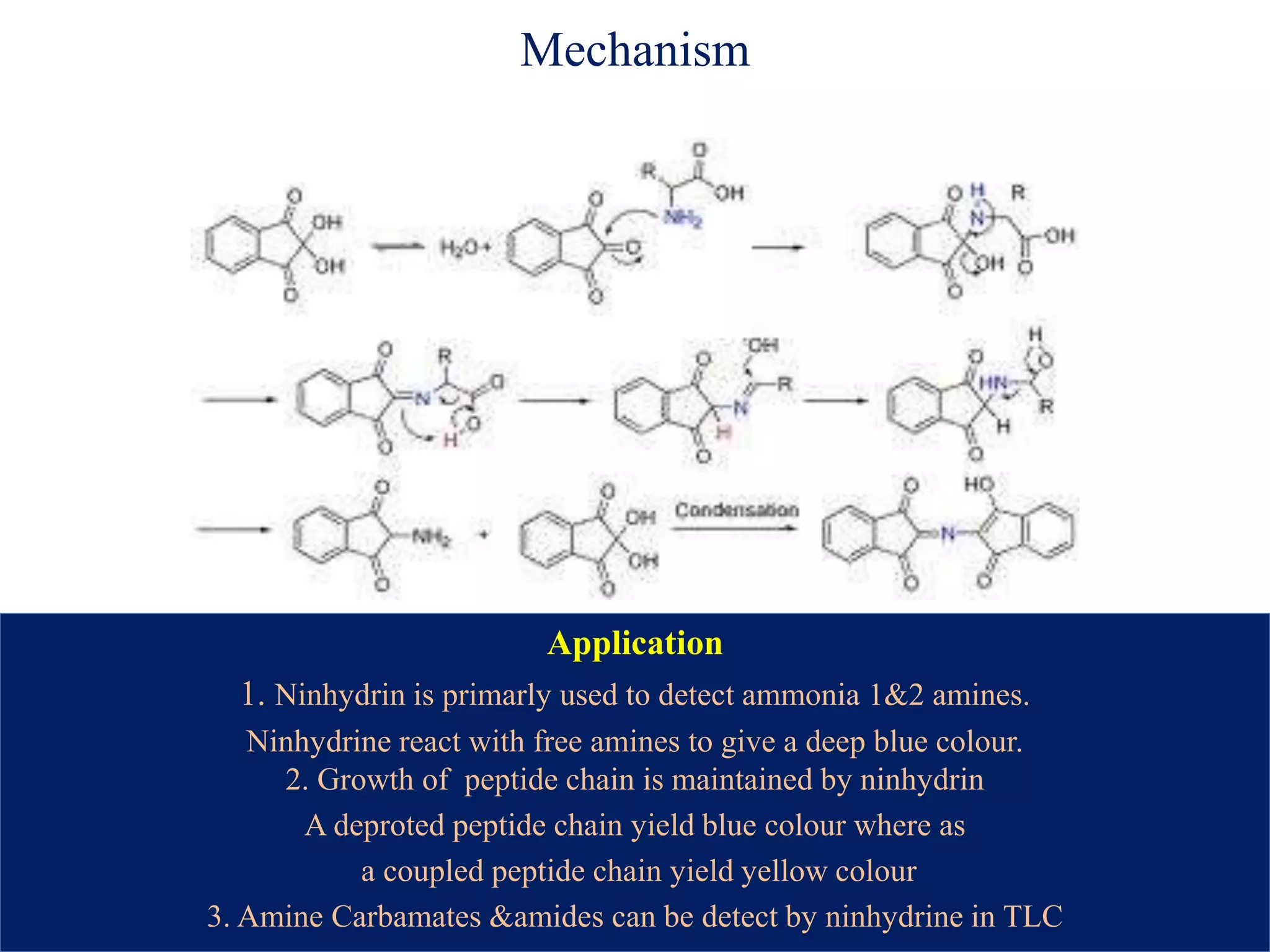
The document discusses various reagents used in pharmaceutical analysis. It begins by classifying reagents based on their reaction mechanisms such as oxidation, condensation, diazotization, and others. Several examples of reagents are provided for each classification including MBTH, Folin-Ciocalteu reagent, PDAB, and 2,4-DNP. Their chemical properties and applications in estimating specific drugs are described. The document concludes by providing an example estimation procedure using MBTH reagent.