Embed presentation
Download to read offline
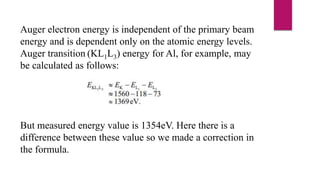
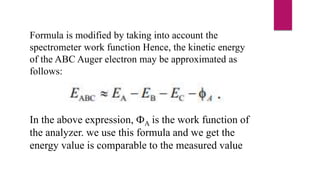

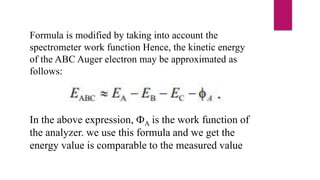

The document discusses Auger electron energy and Auger electron spectroscopy. It explains that Auger electron energy is dependent on atomic energy levels and not the primary beam energy. It also describes modifying the formula for calculating Auger electron energy by accounting for the spectrometer work function to get a value comparable to measured energies. Finally, it notes that Auger electron spectroscopy is used for surface analysis since emitted electrons typically have energies between 50 eV to 3 keV, giving it surface sensitivity.




