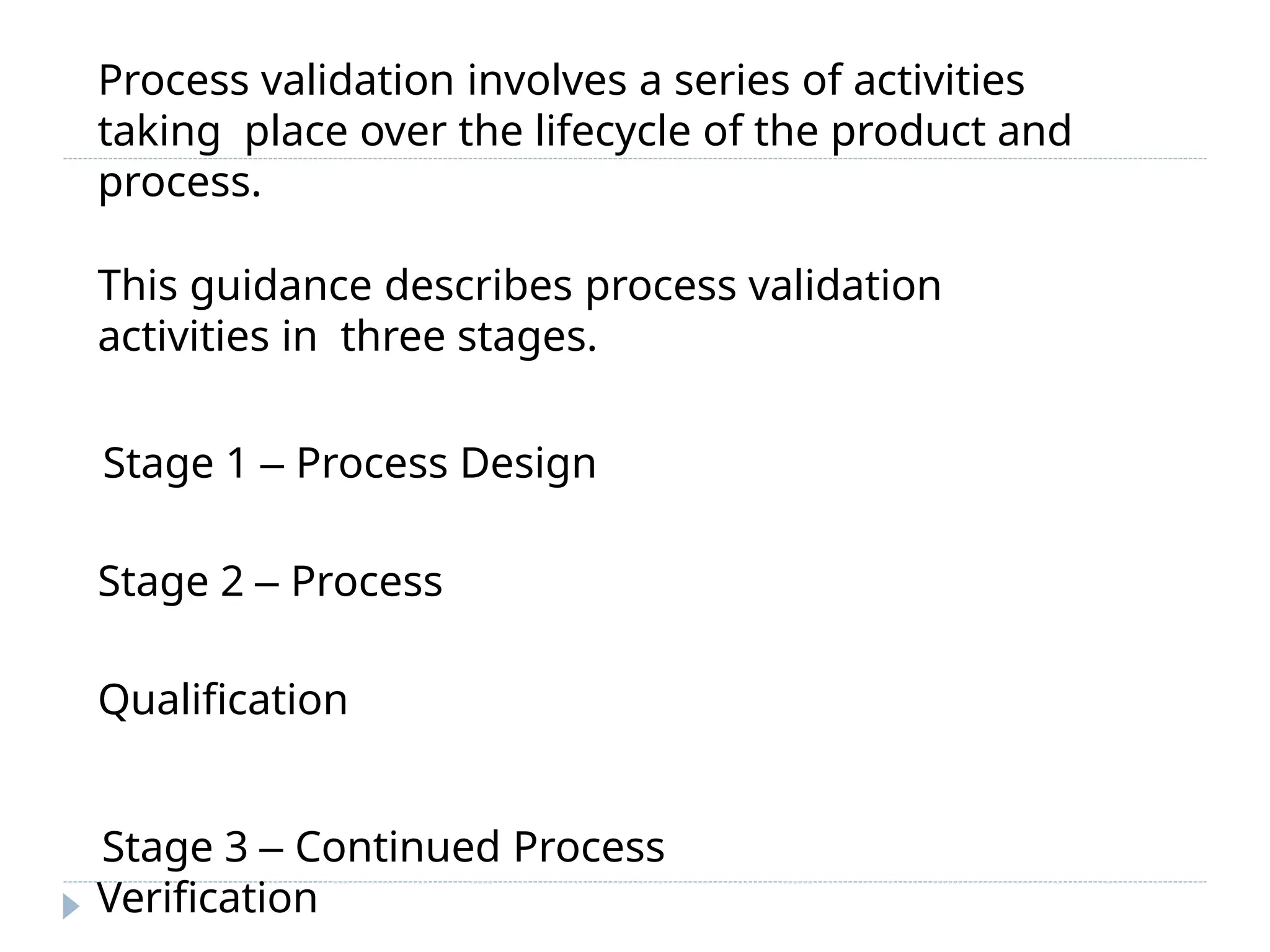
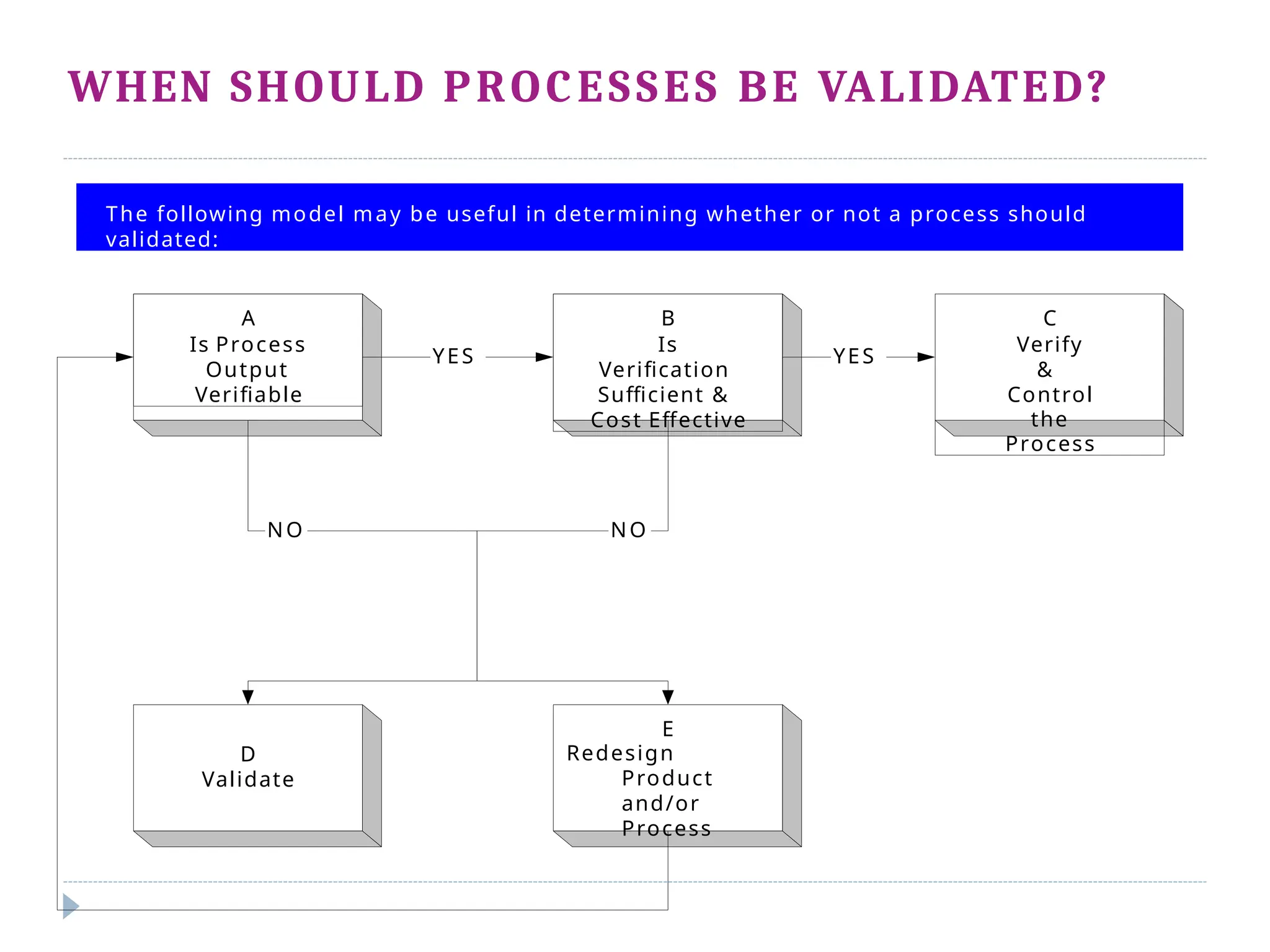
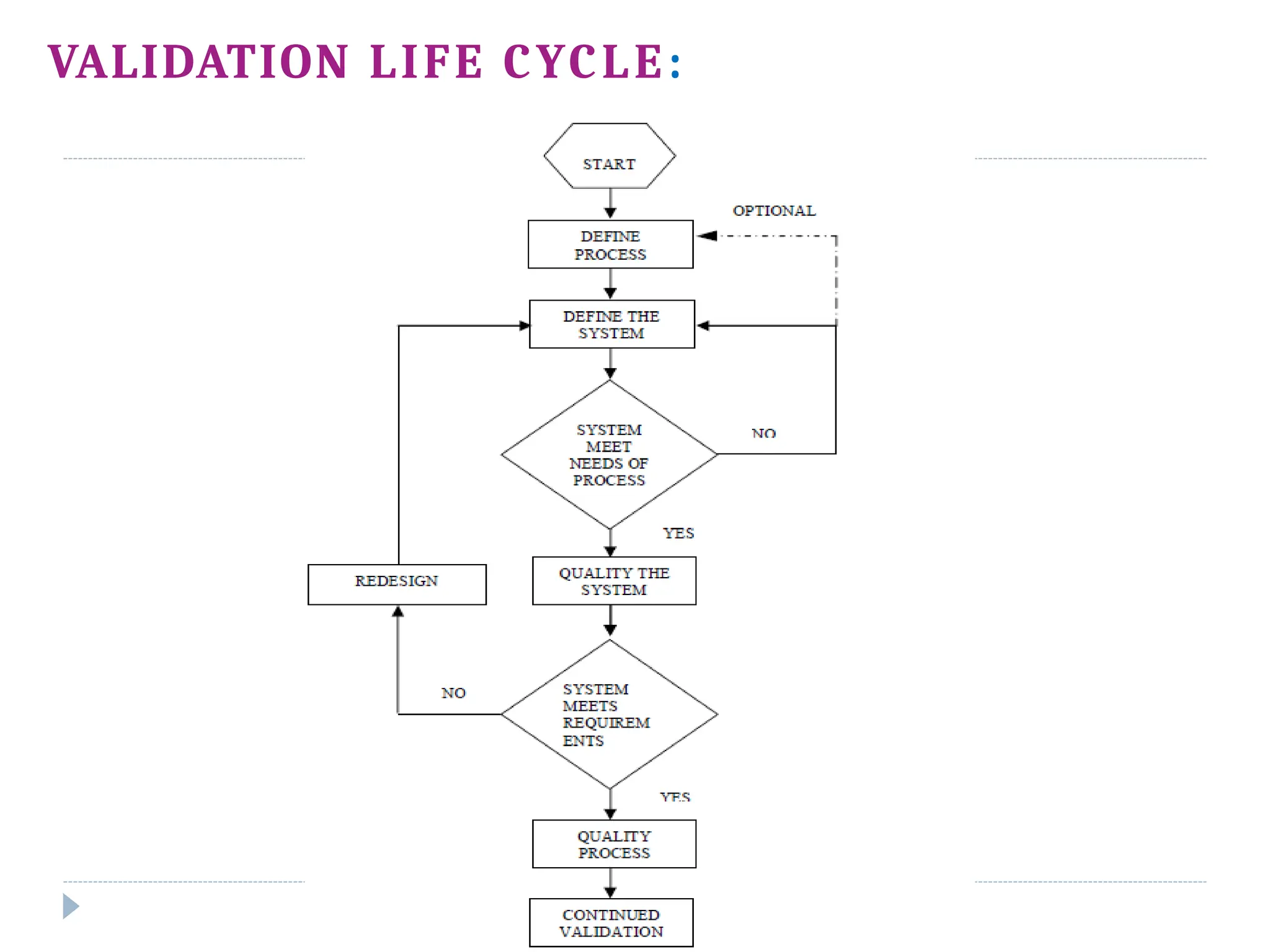
This document outlines the process validation stages in pharmaceutical manufacturing, which include process design, process qualification, and continued process verification. It emphasizes the importance of an integrated team approach, proper documentation, and the evaluation of variations in the manufacturing process. The document also discusses different types of process validation, including prospective, retrospective, and concurrent validations, and highlights their significance in ensuring product quality and compliance with regulations.