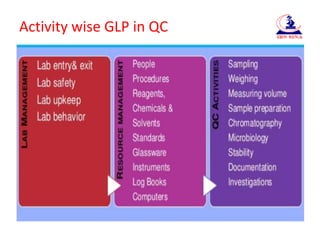
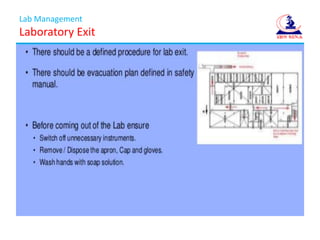
The document discusses Good Laboratory Practices (GLP) for pharmaceutical quality control laboratories. It covers GLP requirements from regulatory authorities like the FDA and WHO. The presentation is divided into sections on lab management, resource management, and quality control activities. Lab management covers topics like laboratory entry and exit procedures, safety, and upkeep. Resource management addresses management of personnel, procedures, reagents, instruments, and documentation. Quality control activities include sampling, chromatography, microbiology, stability testing, and documentation of investigations. Maintaining GLP standards helps ensure quality products for patients and regulatory compliance.