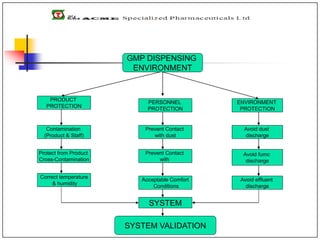
The document discusses requirements for weighing and dispensing operations during pharmaceutical production. It outlines that dispensing should take place in a clean room environment maintained at controlled temperature, humidity, and positive pressure. Specific facilities like dispensing booths, balances, utensils storage, and pass boxes are needed, separated from personnel and material flows. Standard operating procedures and documentation must also be established to validate the dispensing system.