Interpreting energy level diagram
•
3 likes•3,215 views
interpret energy level diagram for exothermic & endothermic reaction
Report
Share
Report
Share
Download to read offline
Recommended
Linear equations in two variables

This presentation include various methods of solving linear equations like substitution, elimination and cross-multiplication method.
Recommended
Linear equations in two variables

This presentation include various methods of solving linear equations like substitution, elimination and cross-multiplication method.
Factoring by grouping

How to factor by grouping
Assessments and examples are available
Can be used by a math teacher in her discussion about factoring
Successive differentiation

Infomatica, as it stands today, is a manifestation of our values, toil, and dedication towards imparting knowledge to the pupils of the society. Visit us: http://www.infomaticaacademy.com/
Solving Quadratic Equations

Provides example on how to solve quadratic equations using extracting square roots, factoring, completing the square and quadratic formula.
Chemical energetics 04

F.Y. B. Sc., Semester -I
Physical Chemistry
Savitribai Phule Pune University, Pune
More Related Content
What's hot
Factoring by grouping

How to factor by grouping
Assessments and examples are available
Can be used by a math teacher in her discussion about factoring
Successive differentiation

Infomatica, as it stands today, is a manifestation of our values, toil, and dedication towards imparting knowledge to the pupils of the society. Visit us: http://www.infomaticaacademy.com/
Solving Quadratic Equations

Provides example on how to solve quadratic equations using extracting square roots, factoring, completing the square and quadratic formula.
What's hot (20)
Similar to Interpreting energy level diagram
Chemical energetics 04

F.Y. B. Sc., Semester -I
Physical Chemistry
Savitribai Phule Pune University, Pune
Chem 16 2 le exam j4 feb 4 2011

A Chem 16 Sample Exam created by J4 for the DLRC Review on February 4, 2011
Ppt 13 R1.2 Energy cycles in reactions.pptx

the ppt is on energy cycles in reactions and deal with Hess's law of constant heat summation where the law of conservation of mass is obeyed. The enthalpy change can be calculated no matter whatever path the reaction has taken place as long as reactants and products are same the enthalpy change can be determined. Thus if enthalpy change of reaction is to be calculated than we can use enthalpy of combustion and formation to calculate it.
Chem 16 2 le answer key j4 feb 4 2011

A Chem 16 Sample Exam Answer Key created by J4 for the DLRC Review on February 4, 2011
Chemical Reactions: Thermochemistry

Lecture materials for the Introductory Chemistry course for Forensic Scientists, University of Lincoln, UK. See http://forensicchemistry.lincoln.ac.uk/ for more details.
Similar to Interpreting energy level diagram (20)
More from sweemoi khor
Chemistry SPM 2012 Paper 1 Question Analysis

Question Analysis for Paper 1 SPM 2012 according to topics
Operational Definition of Reactivity of Alkali Metals with oxygen

How to define operationally for the reactivity of alkali metals towards oxygen
Question Analysis for Paper 3 Trial Chemistry 2011 from Different States

It provides a clear analysis of questions in Paper 3 Trial SPM 2011 from different state
Heat of combustion of Various Alcohols

Definition of heat of combustion, method to determine heat of combustion and compare heat of combustion between various alcohols
More from sweemoi khor (20)
Question Analysis for Paper 3 Trial Chemistry 2012 from Different States

Question Analysis for Paper 3 Trial Chemistry 2012 from Different States
Operational Definition of Reactivity of Alkali Metals with oxygen

Operational Definition of Reactivity of Alkali Metals with oxygen
Question Analysis for Paper 3 Trial Chemistry 2011 from Different States

Question Analysis for Paper 3 Trial Chemistry 2011 from Different States
Interpreting energy level diagram
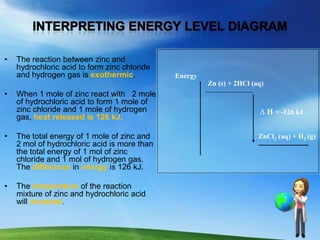
- 1. • The reaction between zinc and hydrochloric acid to form zinc chloride and hydrogen gas is exothermic. Energy Zn (s) + 2HCl (aq) • When 1 mole of zinc react with 2 mole of hydrochloric acid to form 1 mole of zinc chloride and 1 mole of hydrogen ∆ H = -126 kJ gas, heat released is 126 kJ. • The total energy of 1 mole of zinc and ZnCl2 (aq) + H2 (g) 2 mol of hydrochloric acid is more than the total energy of 1 mol of zinc chloride and 1 mol of hydrogen gas. The difference in energy is 126 kJ. • The temperature of the reaction mixture of zinc and hydrochloric acid will increase.
- 2. • The reaction between nitrogen and oxygen to form nitrogen dioxide is endothermic. Energy 2NO2 (g) • When 1 mole of nitrogen react with 2 mole of oxygen to form 2 mole of nitrogen dioxide, heat ∆ H = +66 kJ absorbed is 66 kJ. • The total energy of 2 mole of N2 (g) + 2O2 (g) nitrogen dioxide is more than the total energy of 1 mol of nitrogen chloride and 2 mol of oxygen. The difference in energy is 66kJ. • The temperature of the reaction mixture of zinc and hydrochloric acid will decrease.