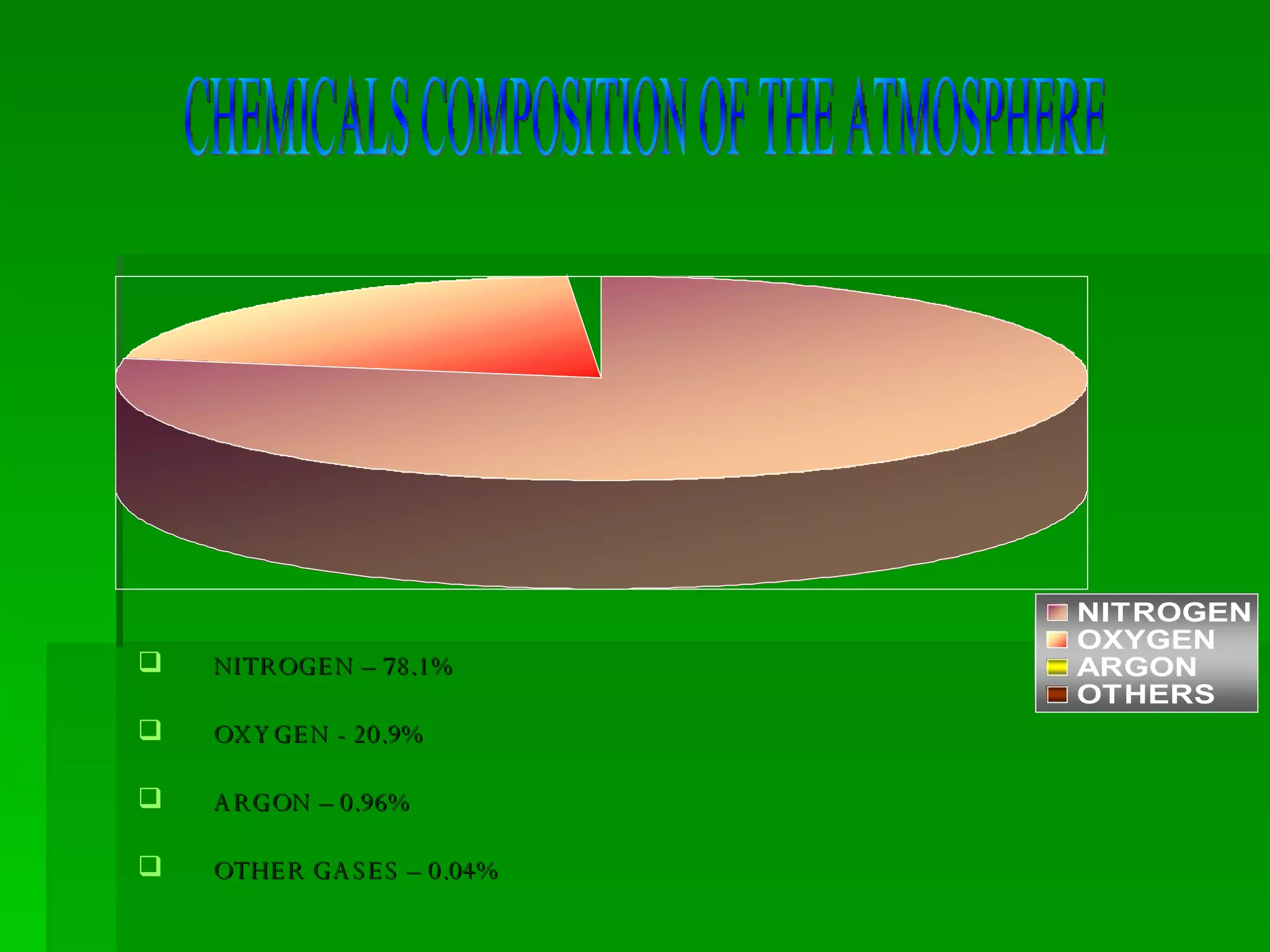
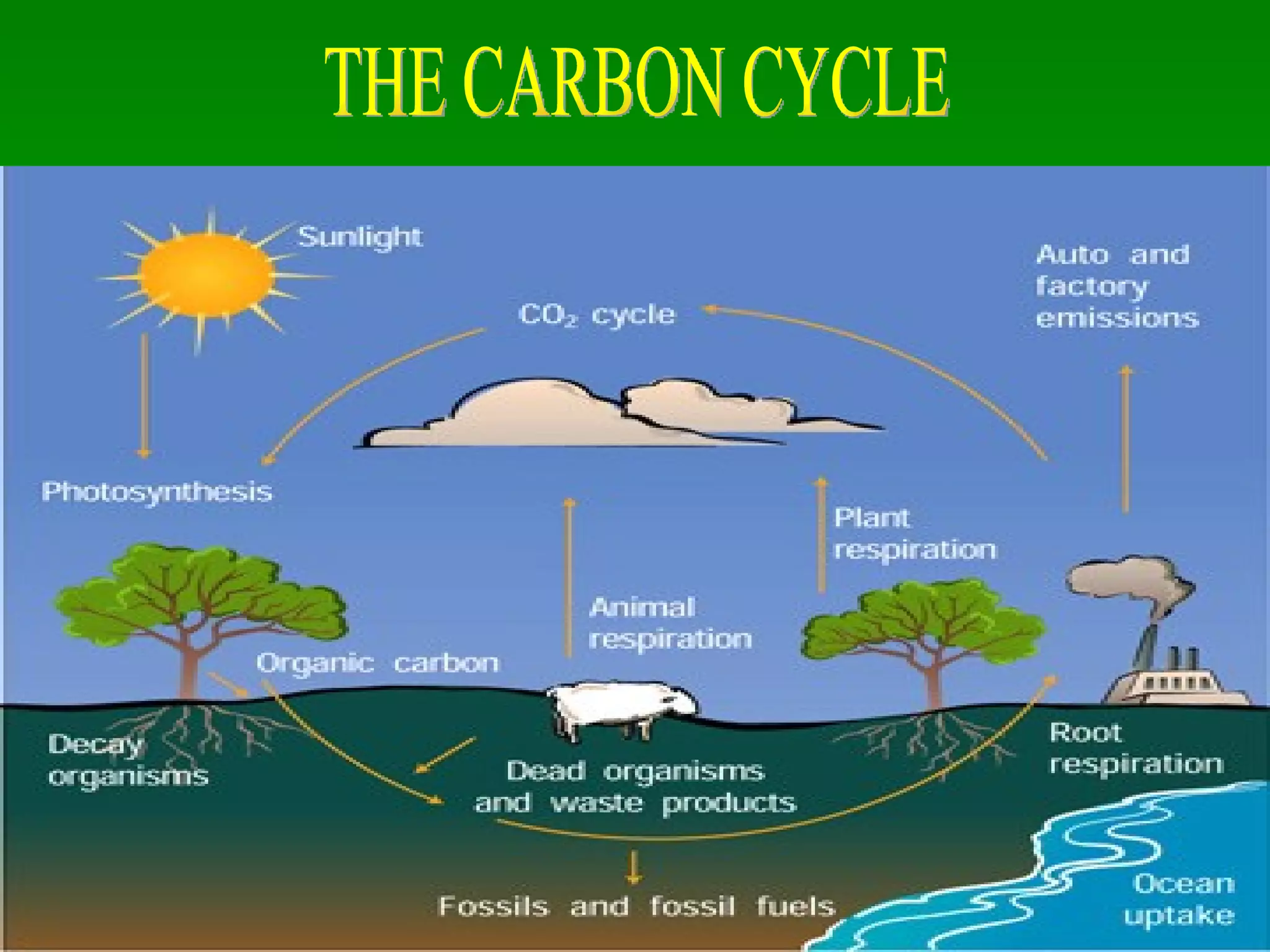
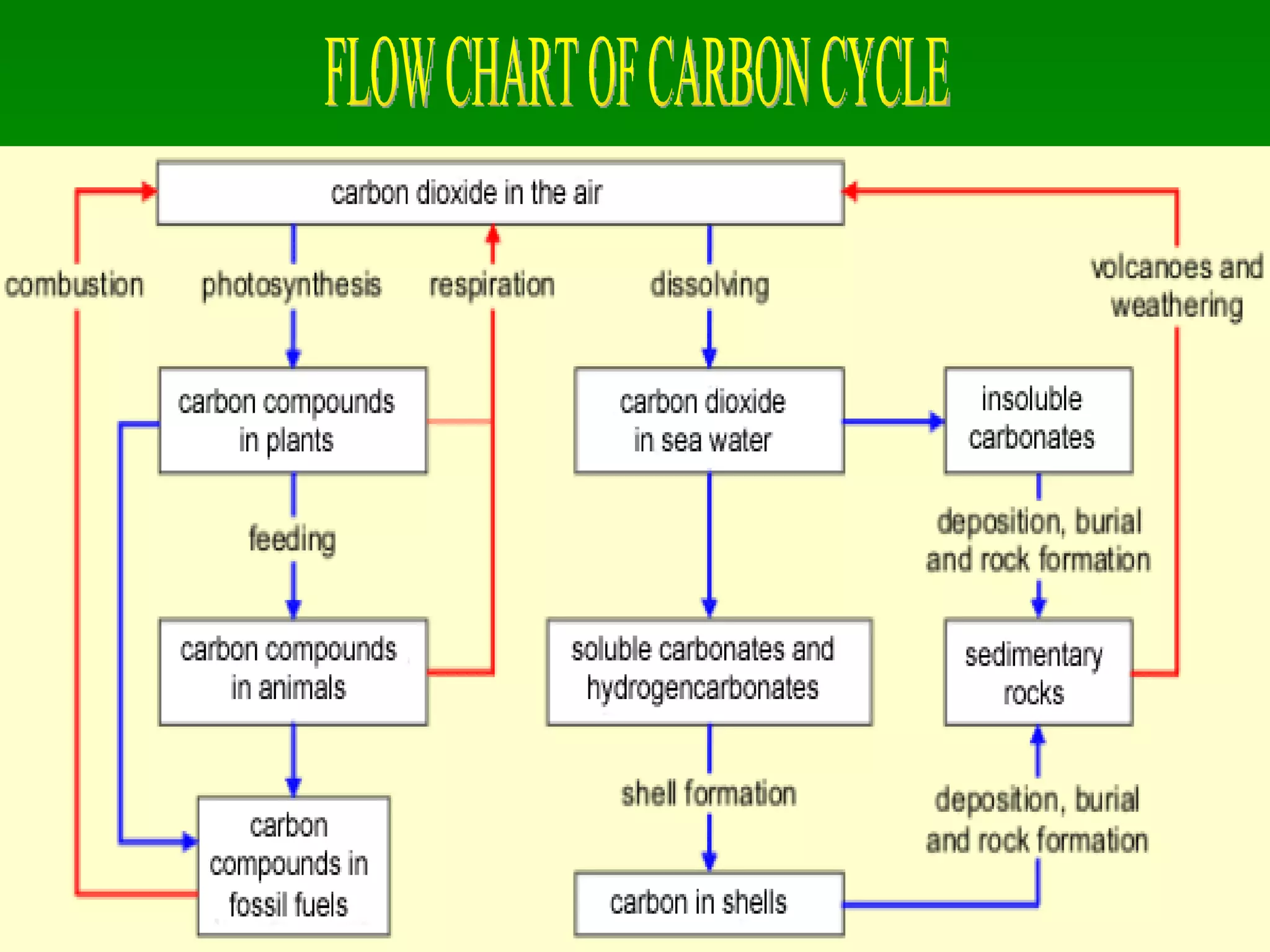
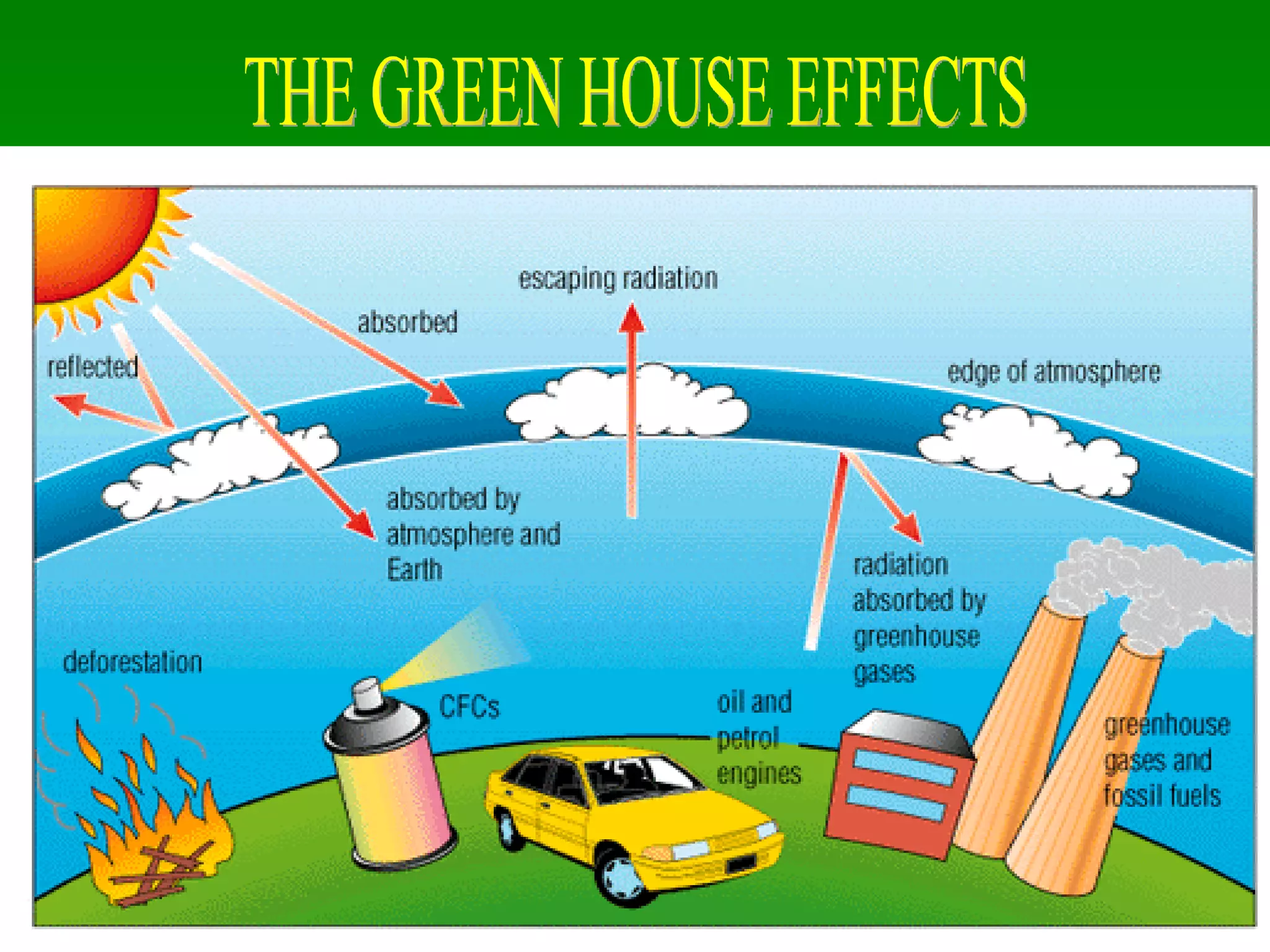
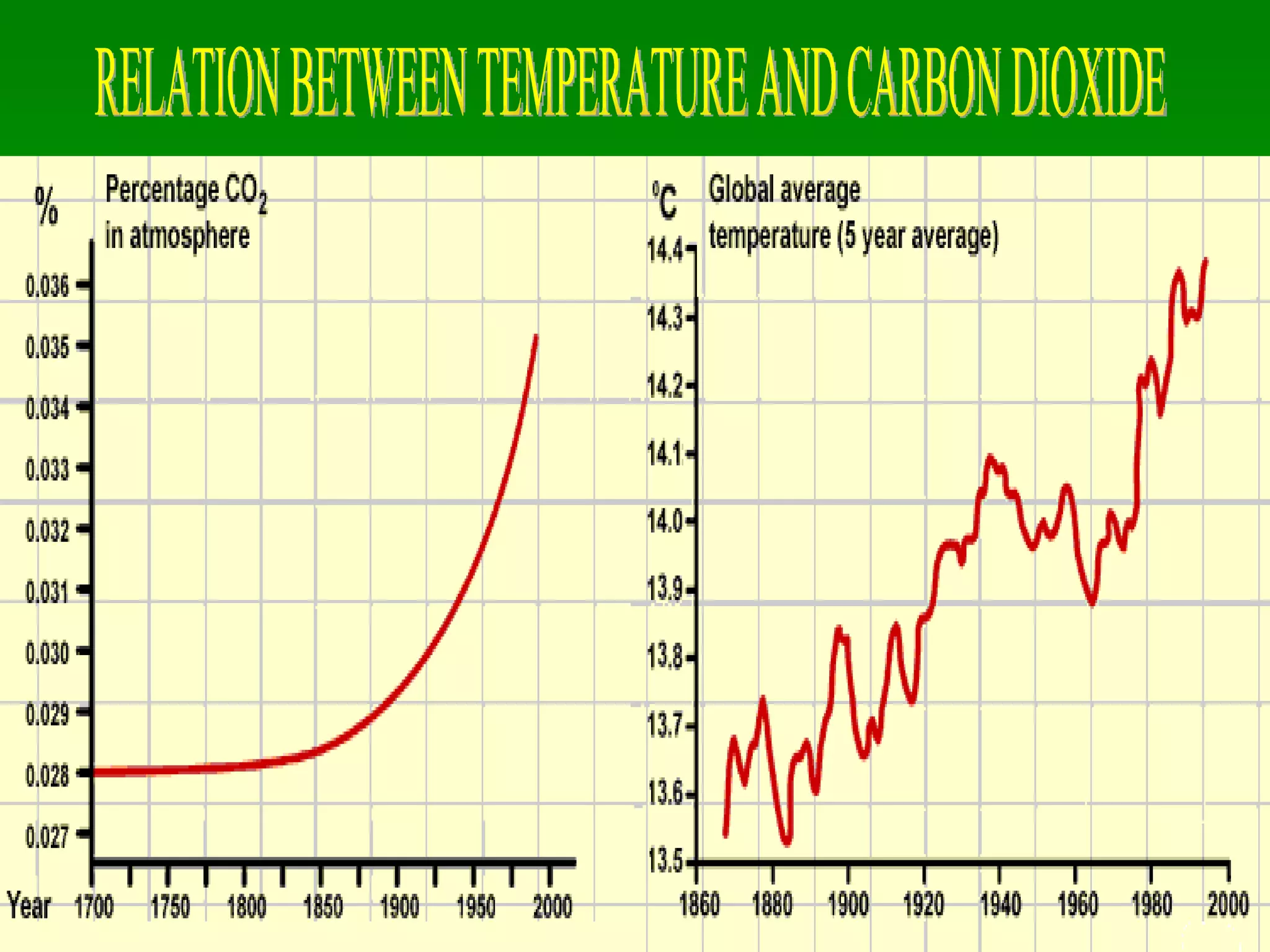
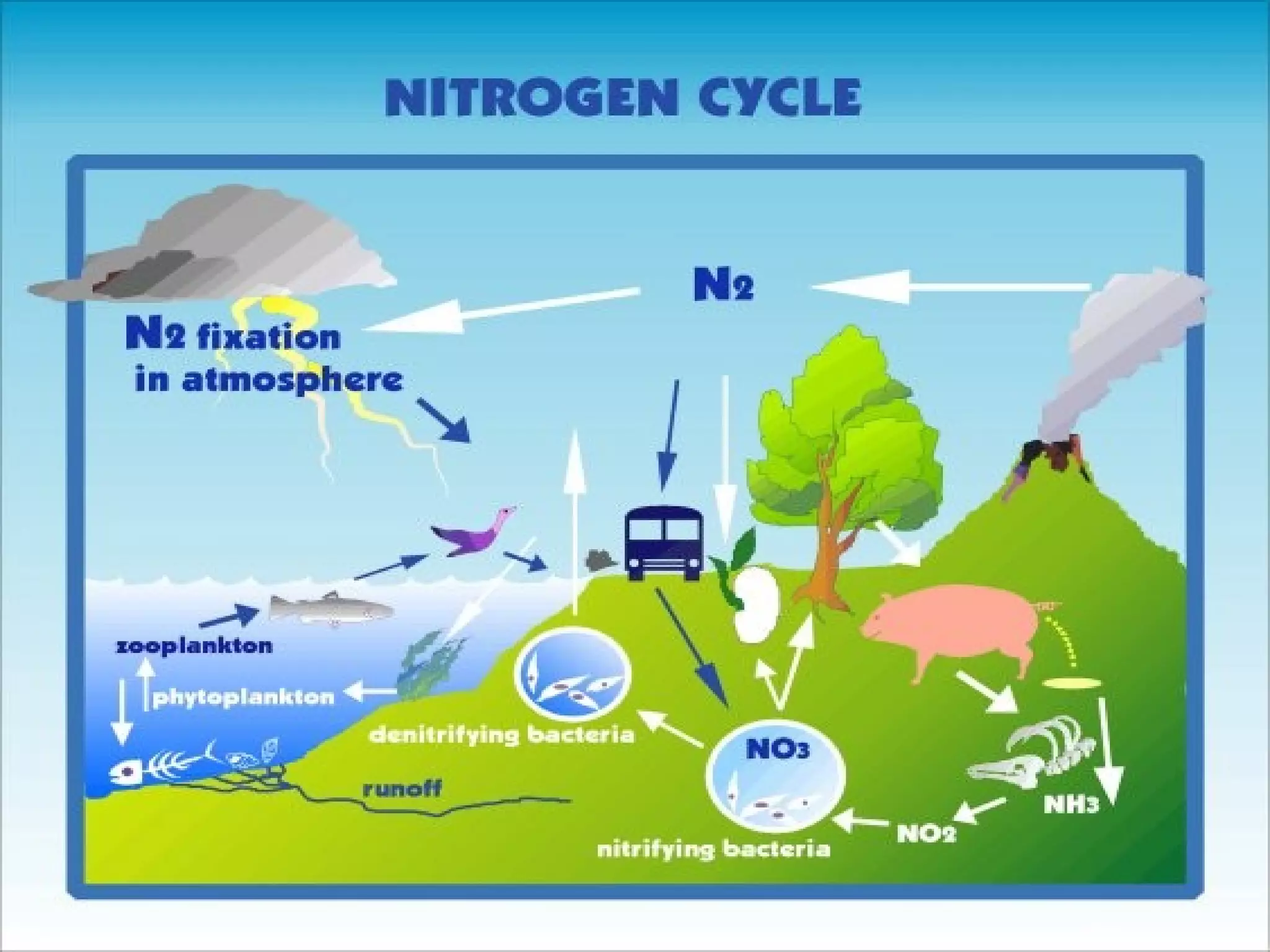
The document discusses the carbon and nitrogen cycles. The carbon cycle involves carbon dioxide being absorbed by plants through photosynthesis and released through respiration and decay. Carbon is also stored long-term in fossil fuels, limestone, and other carbonate rocks. The nitrogen cycle similarly involves nitrogen being fixed from the atmosphere by lightning and bacteria, entering ecosystems, and being released through decay. Both cycles are impacted by human activities like fossil fuel use and fertilizer production.