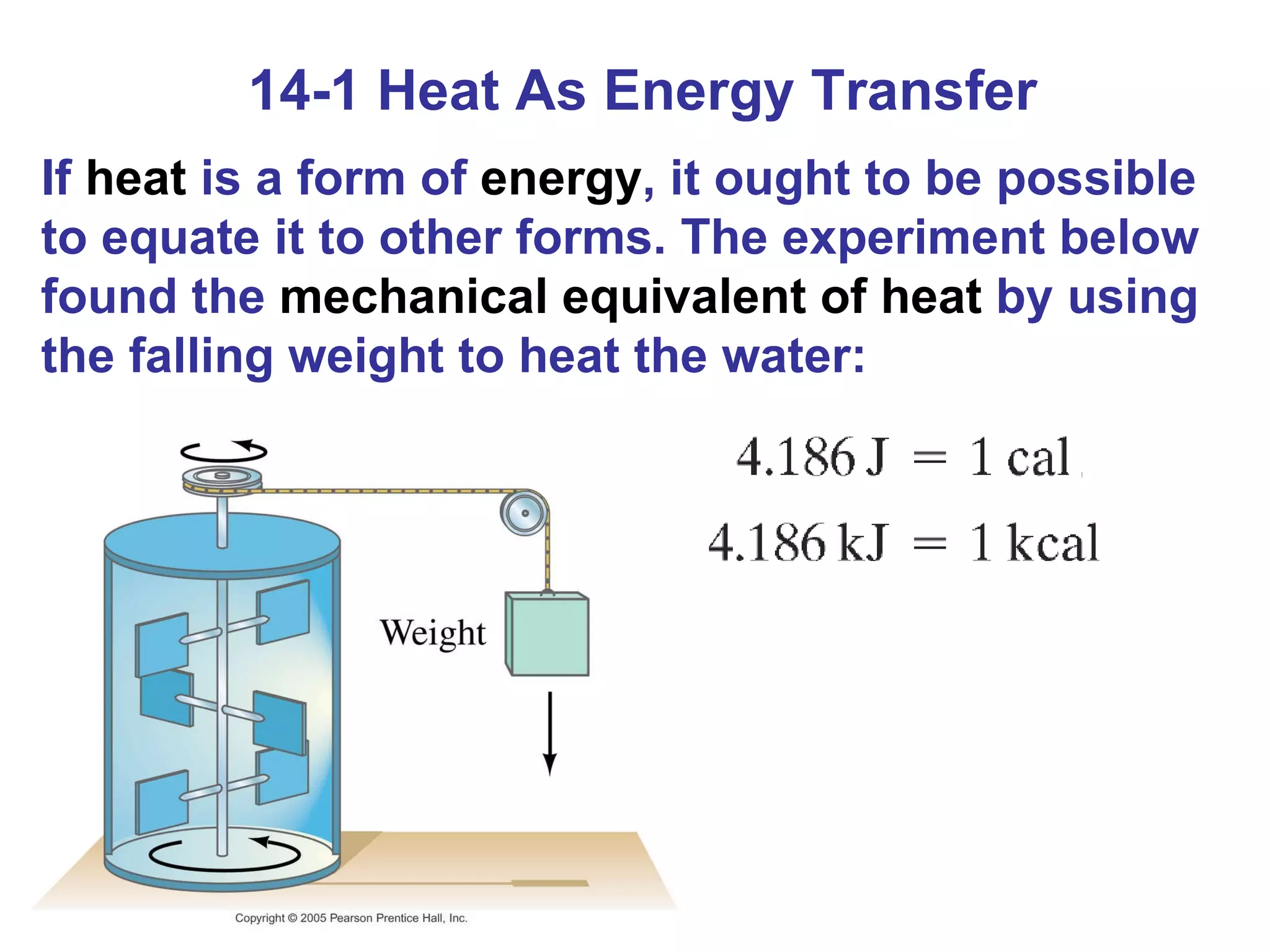
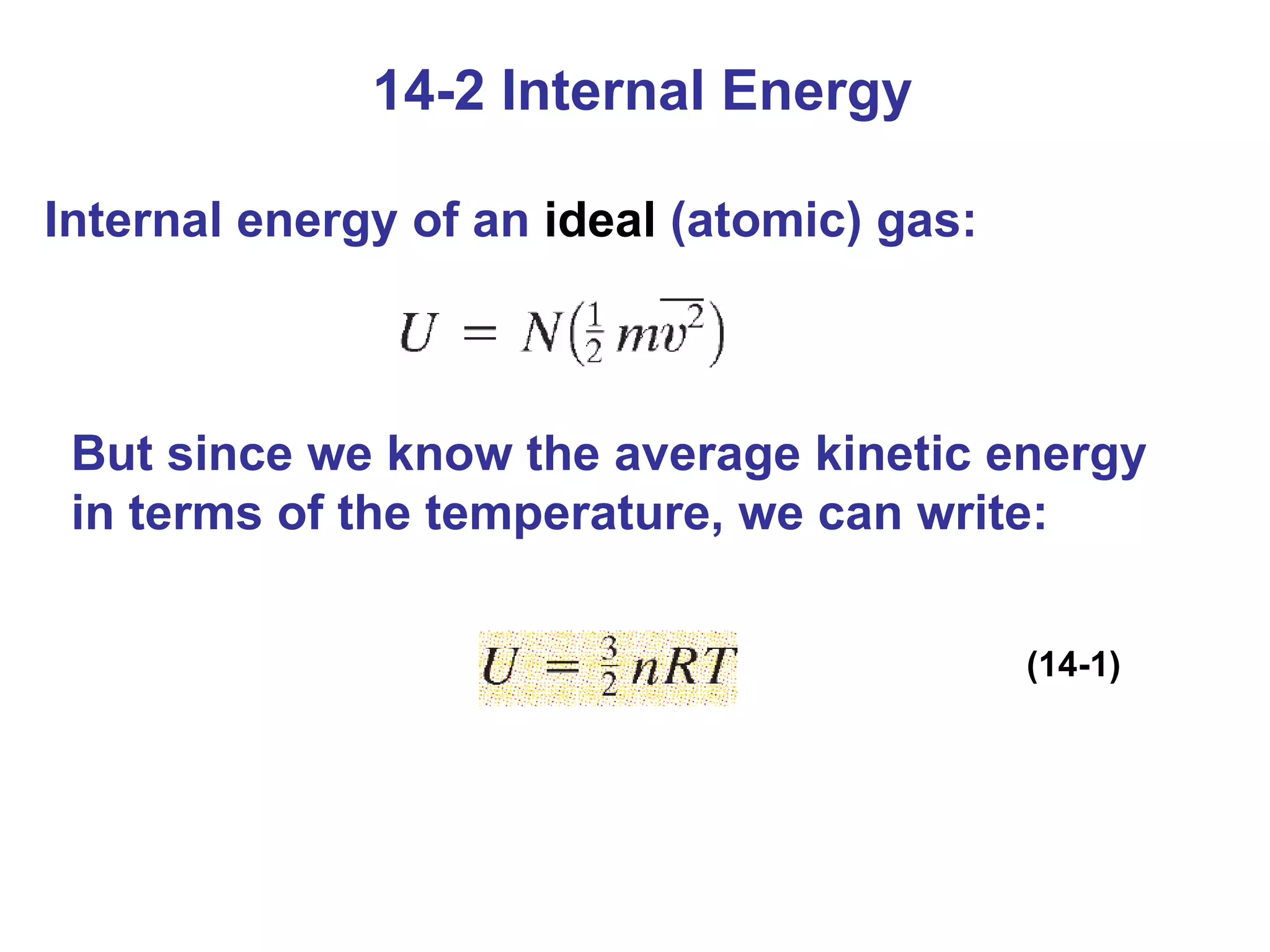
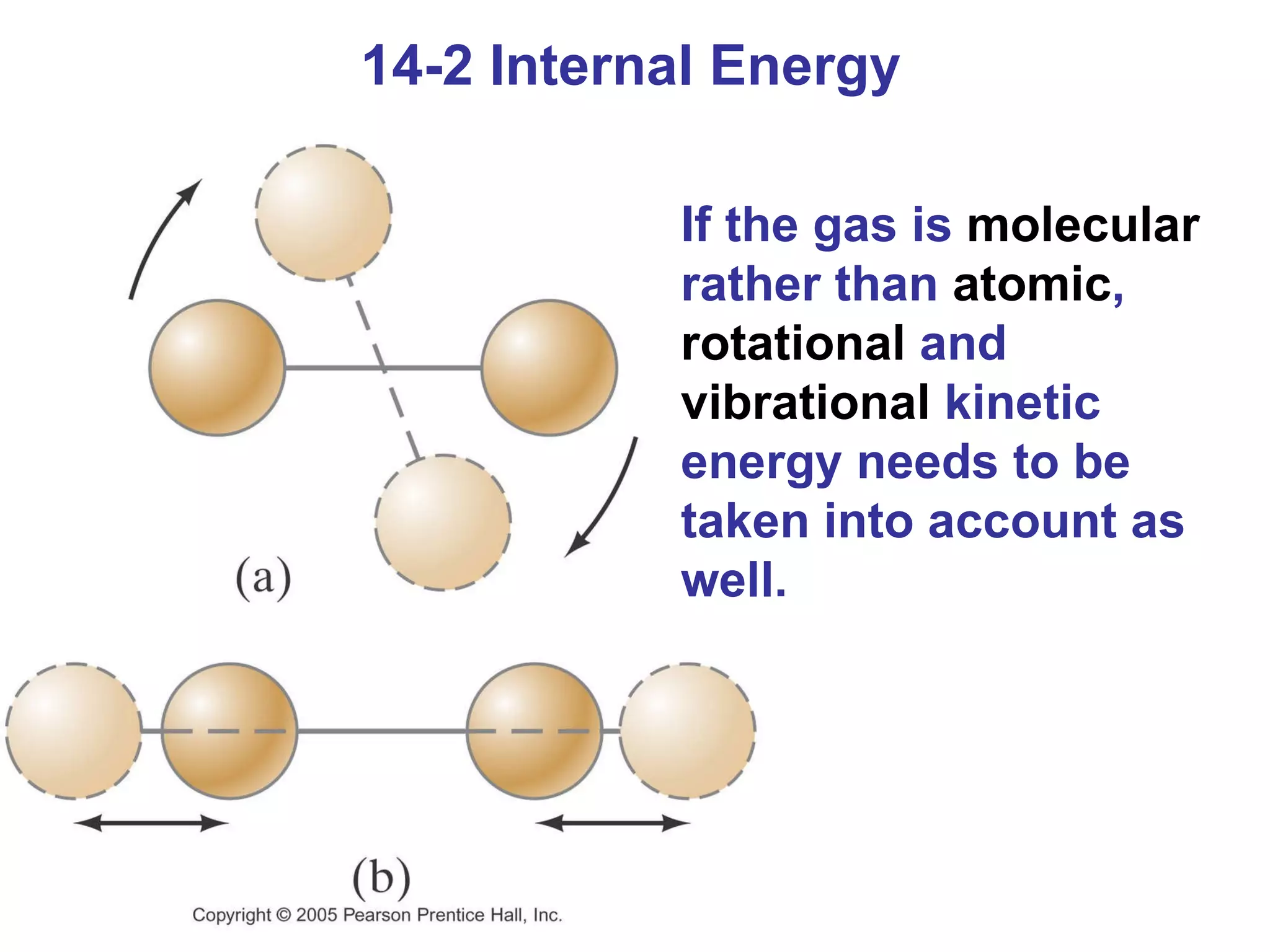
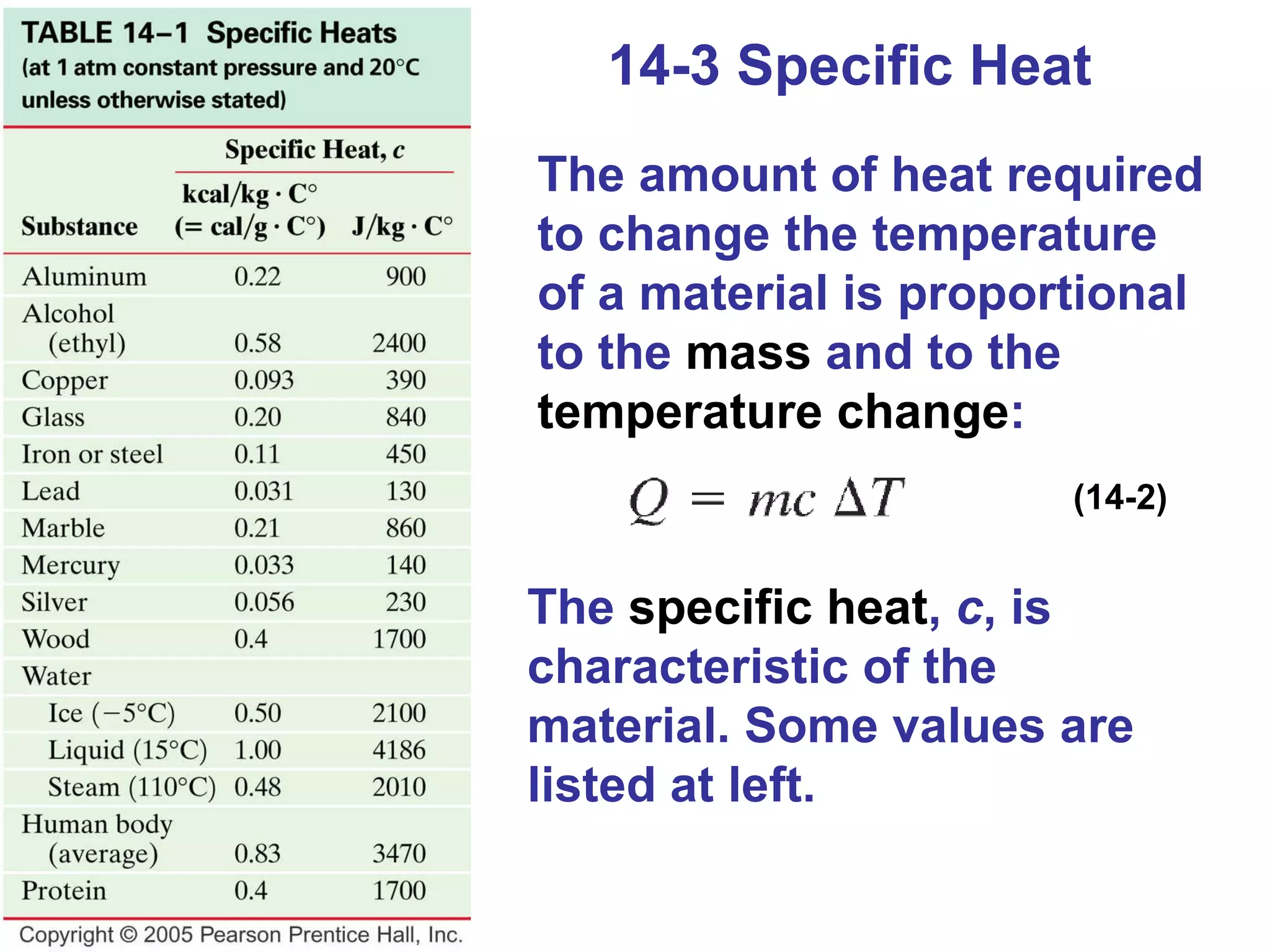
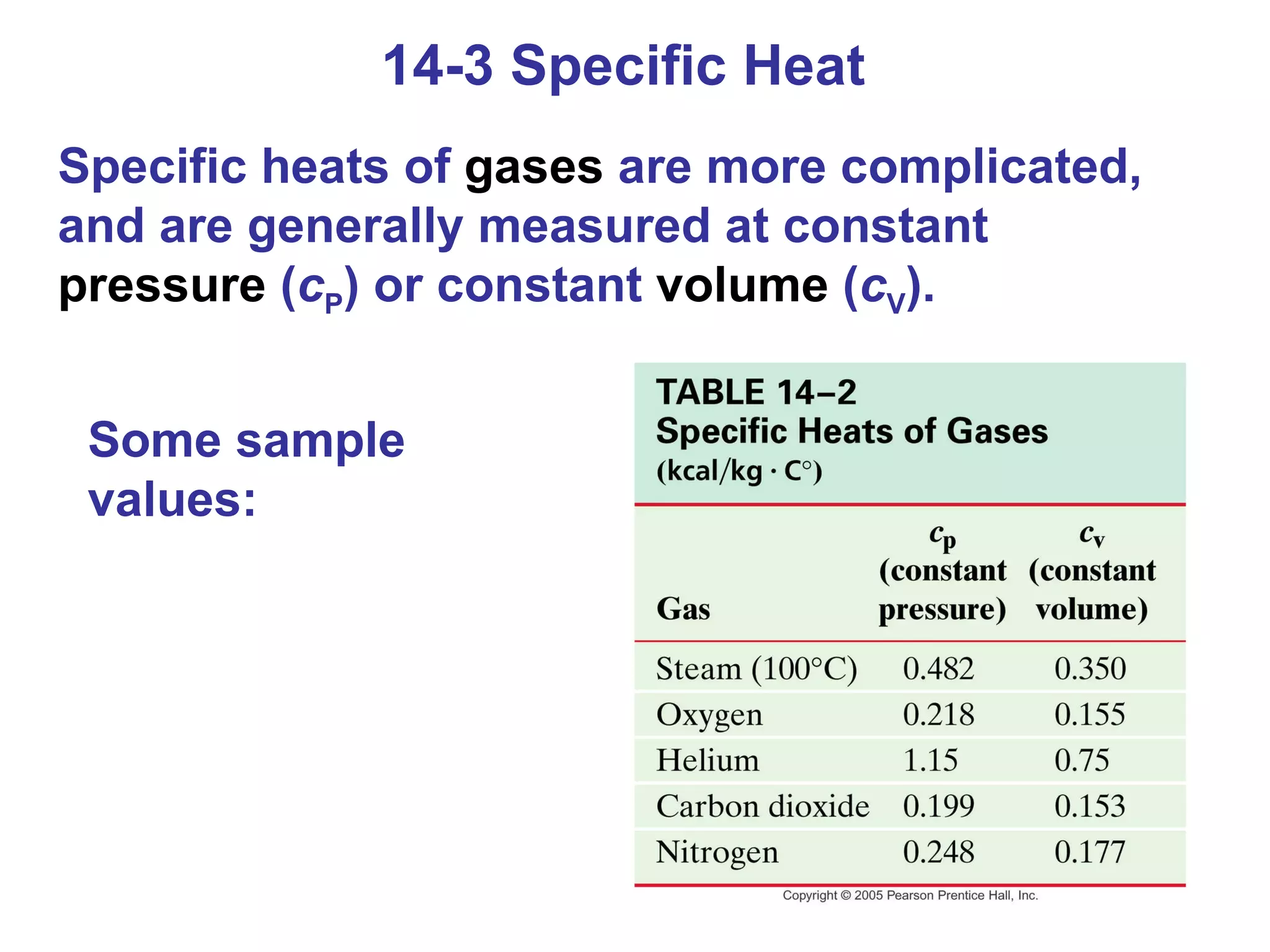
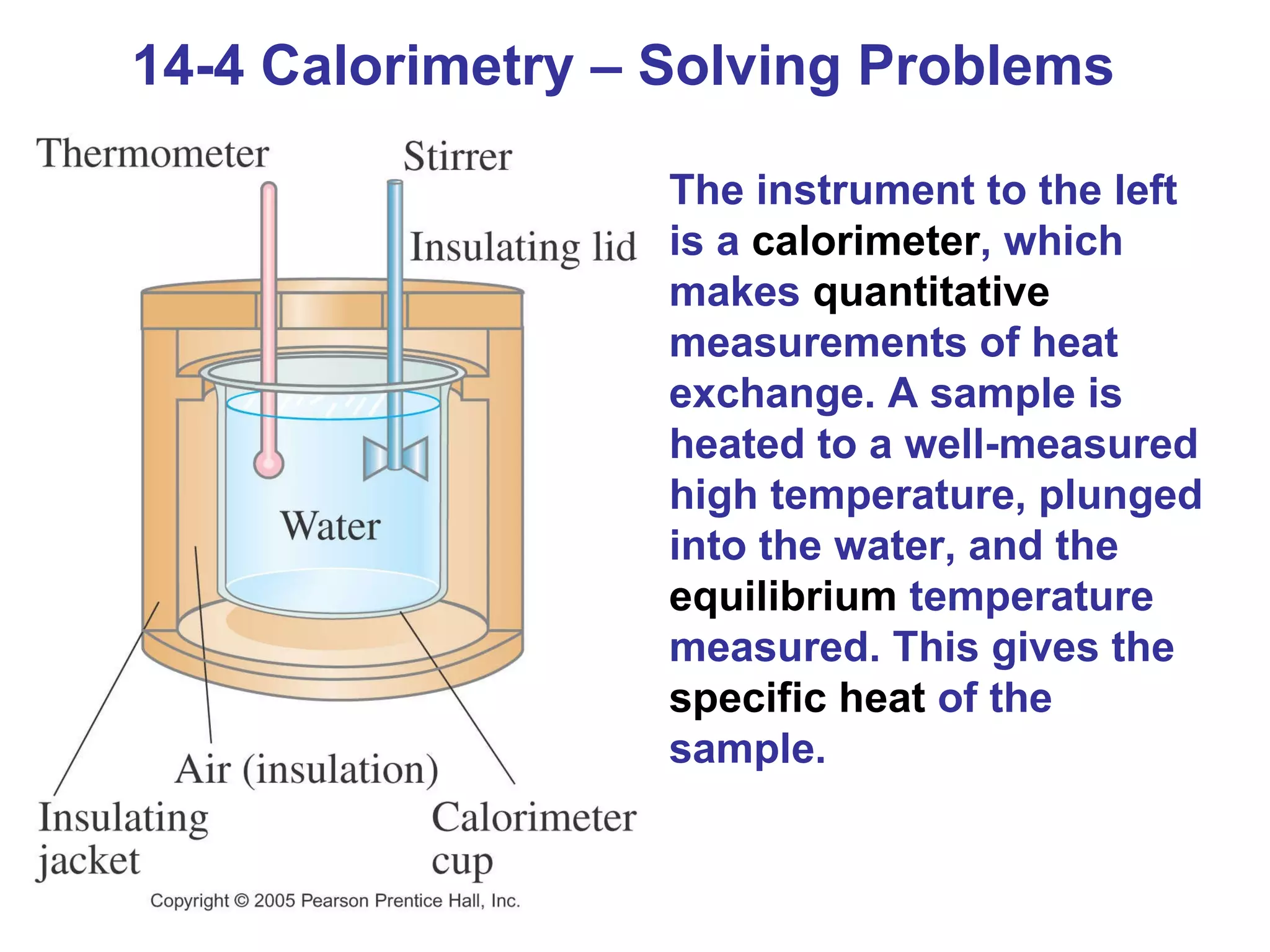
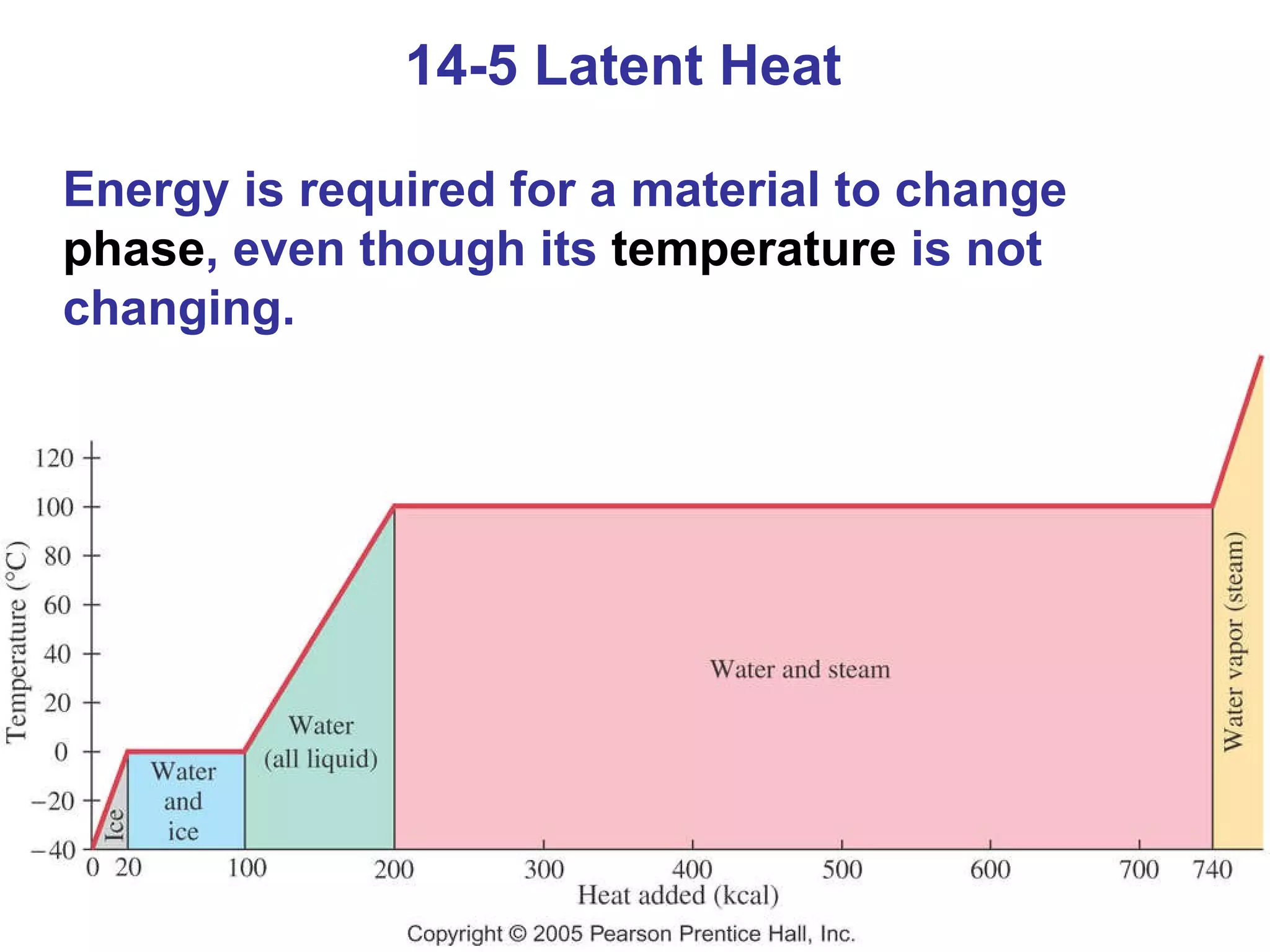
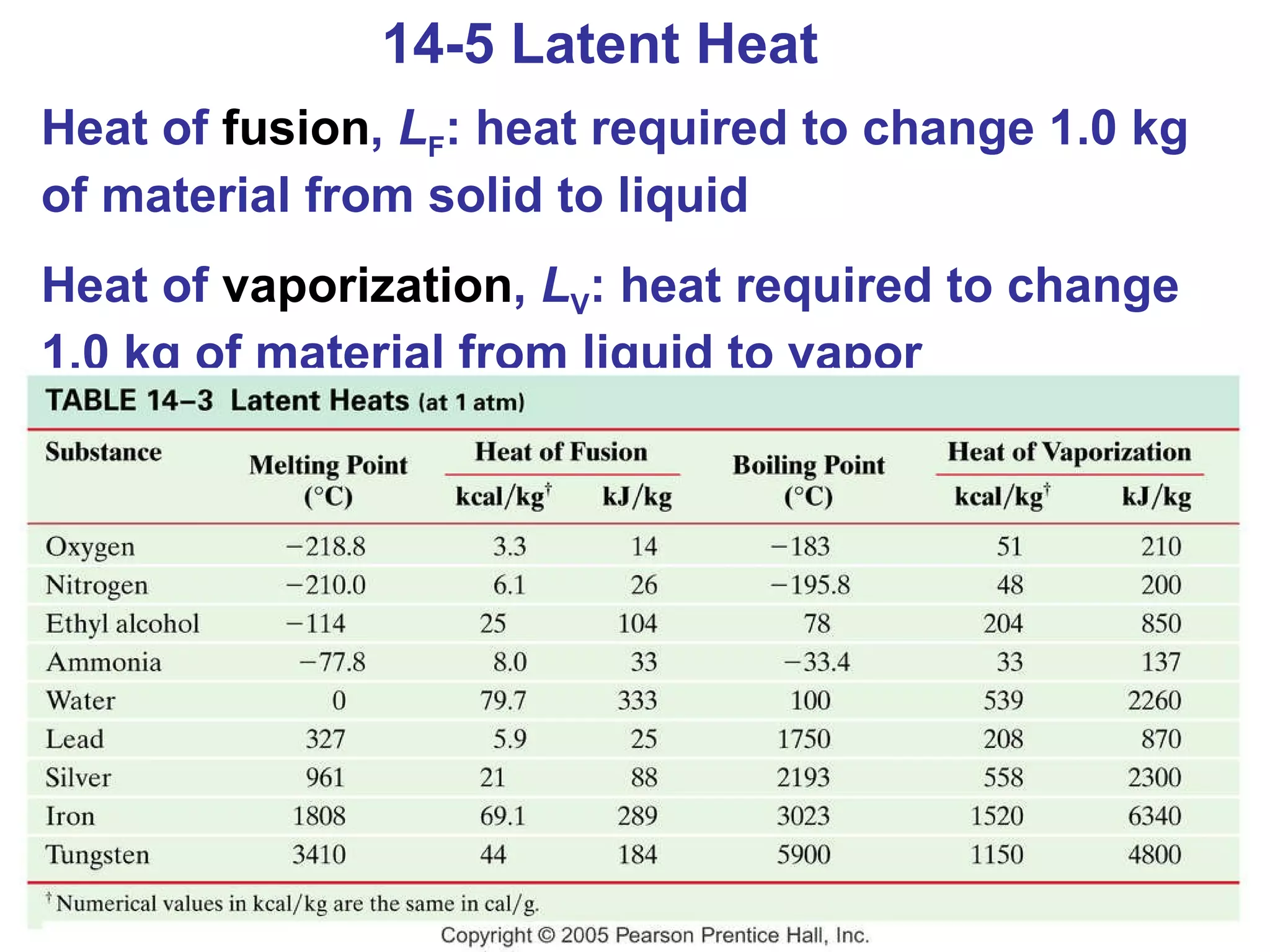
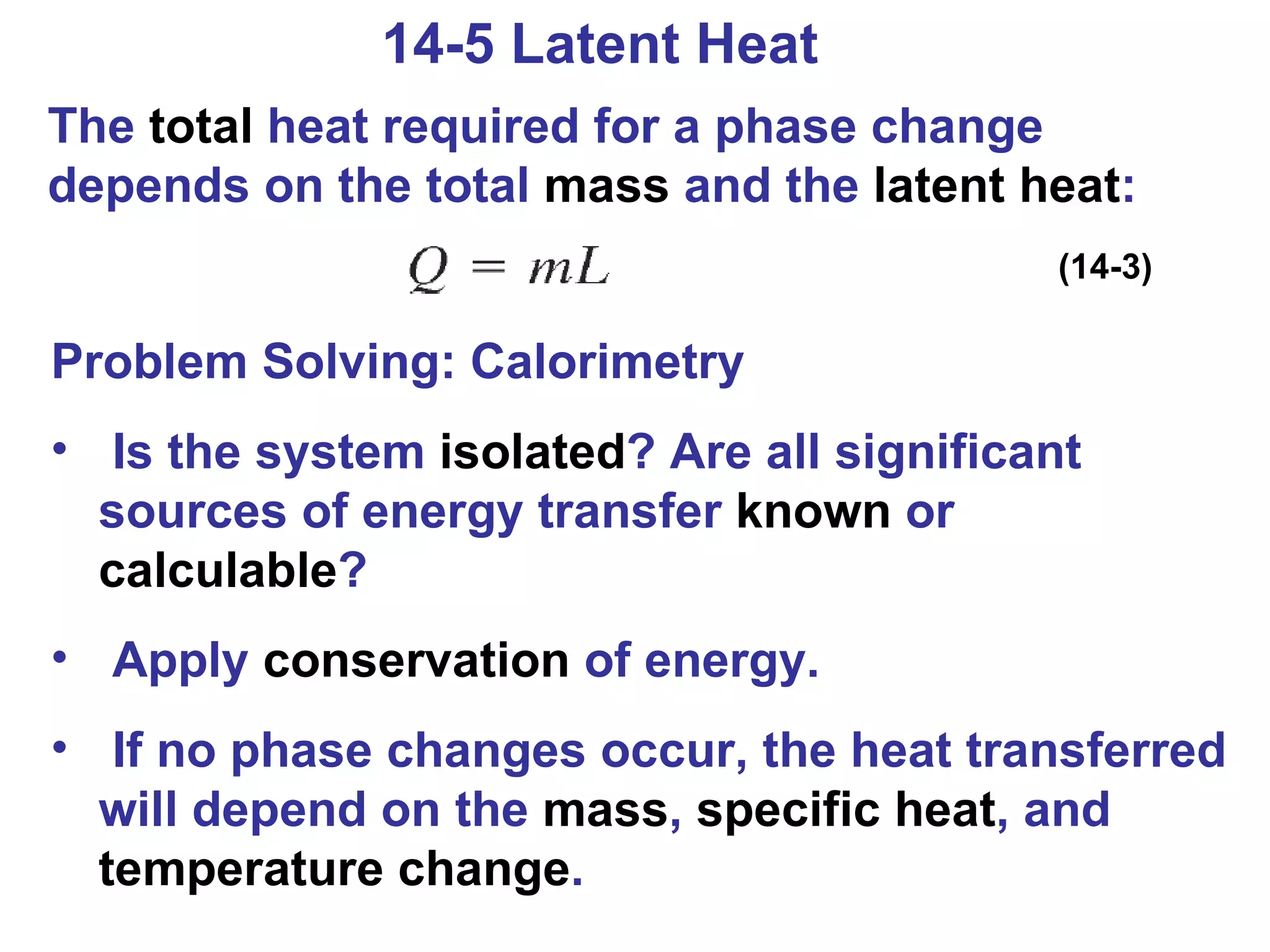
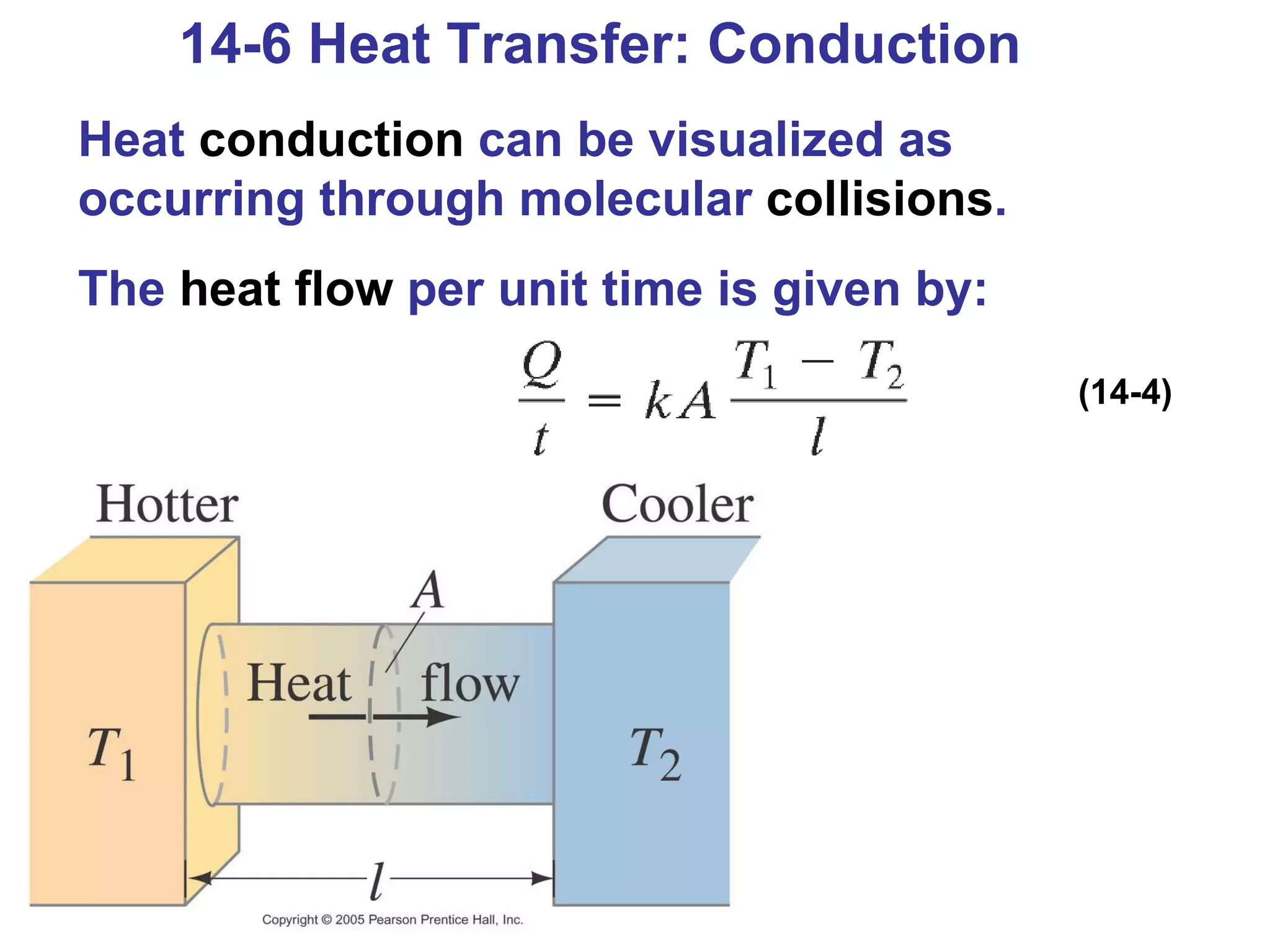
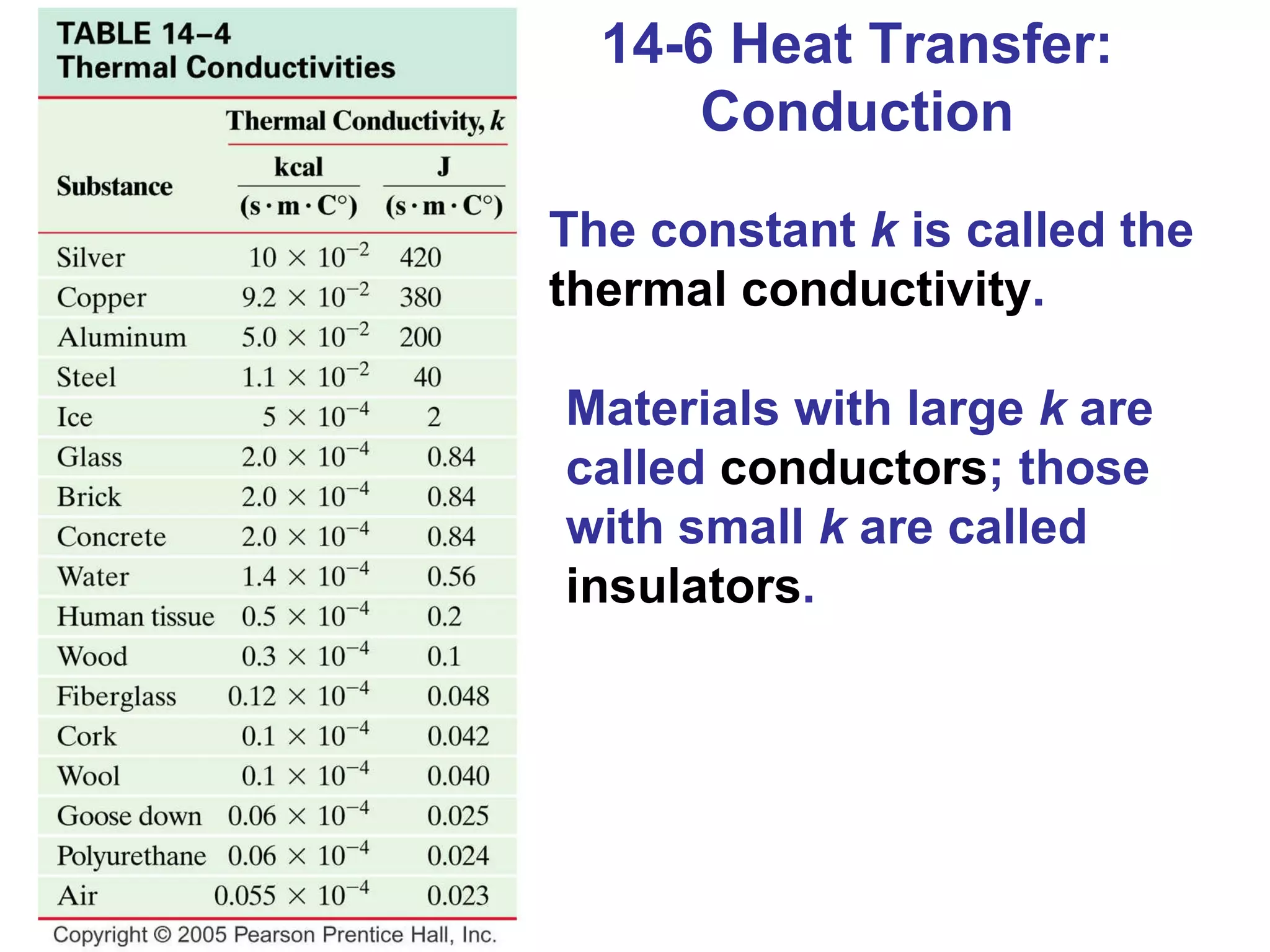
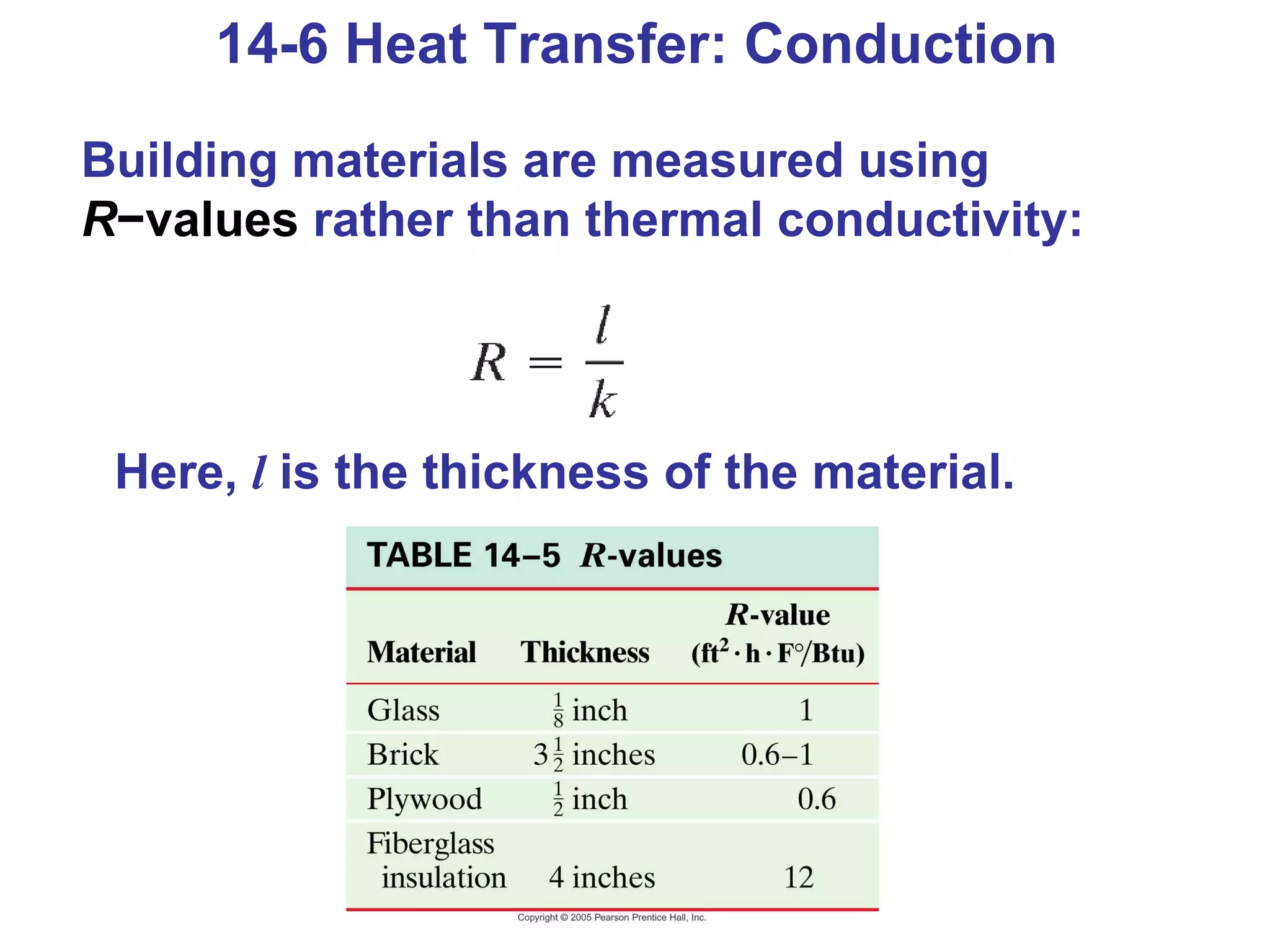
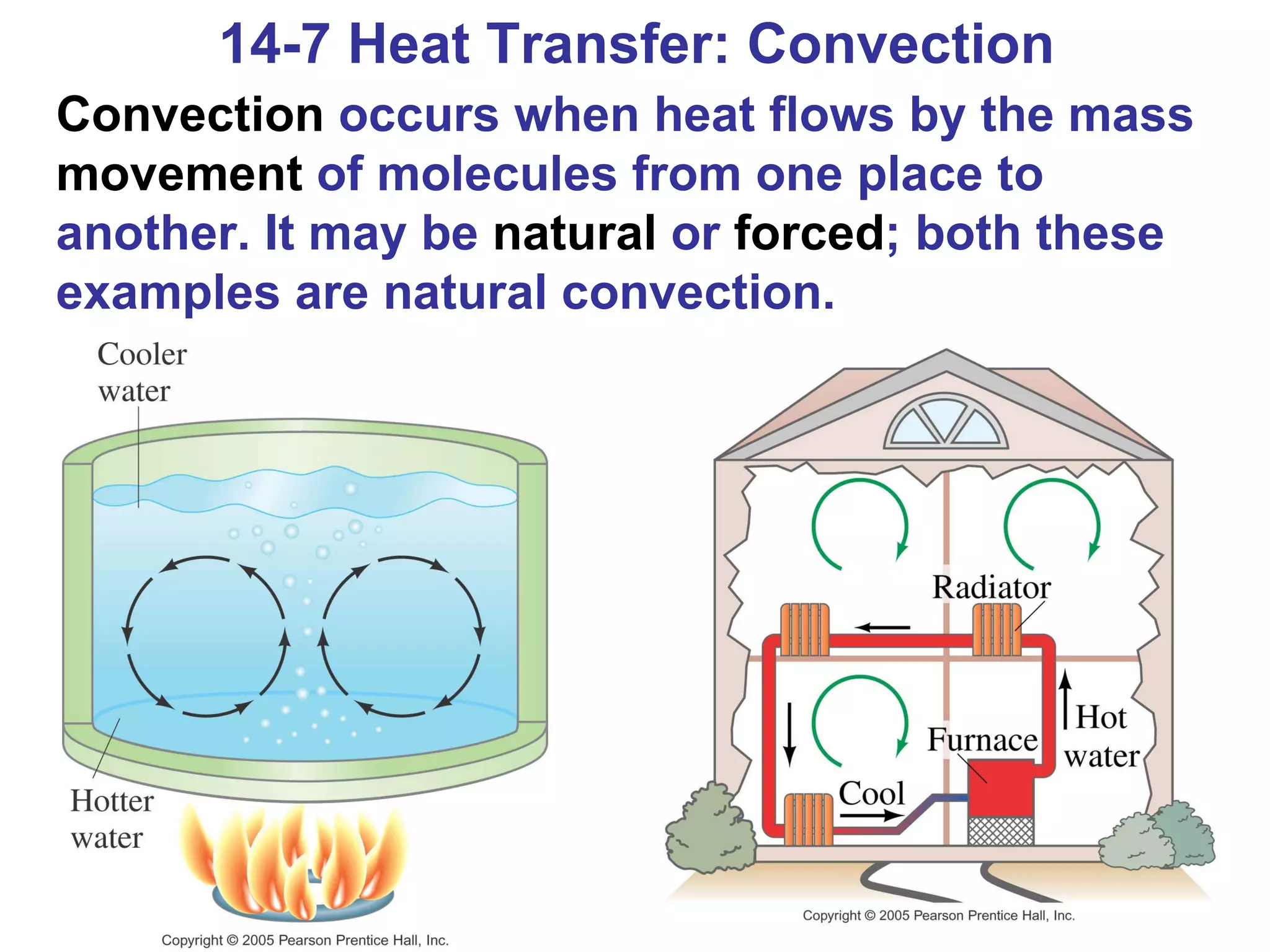
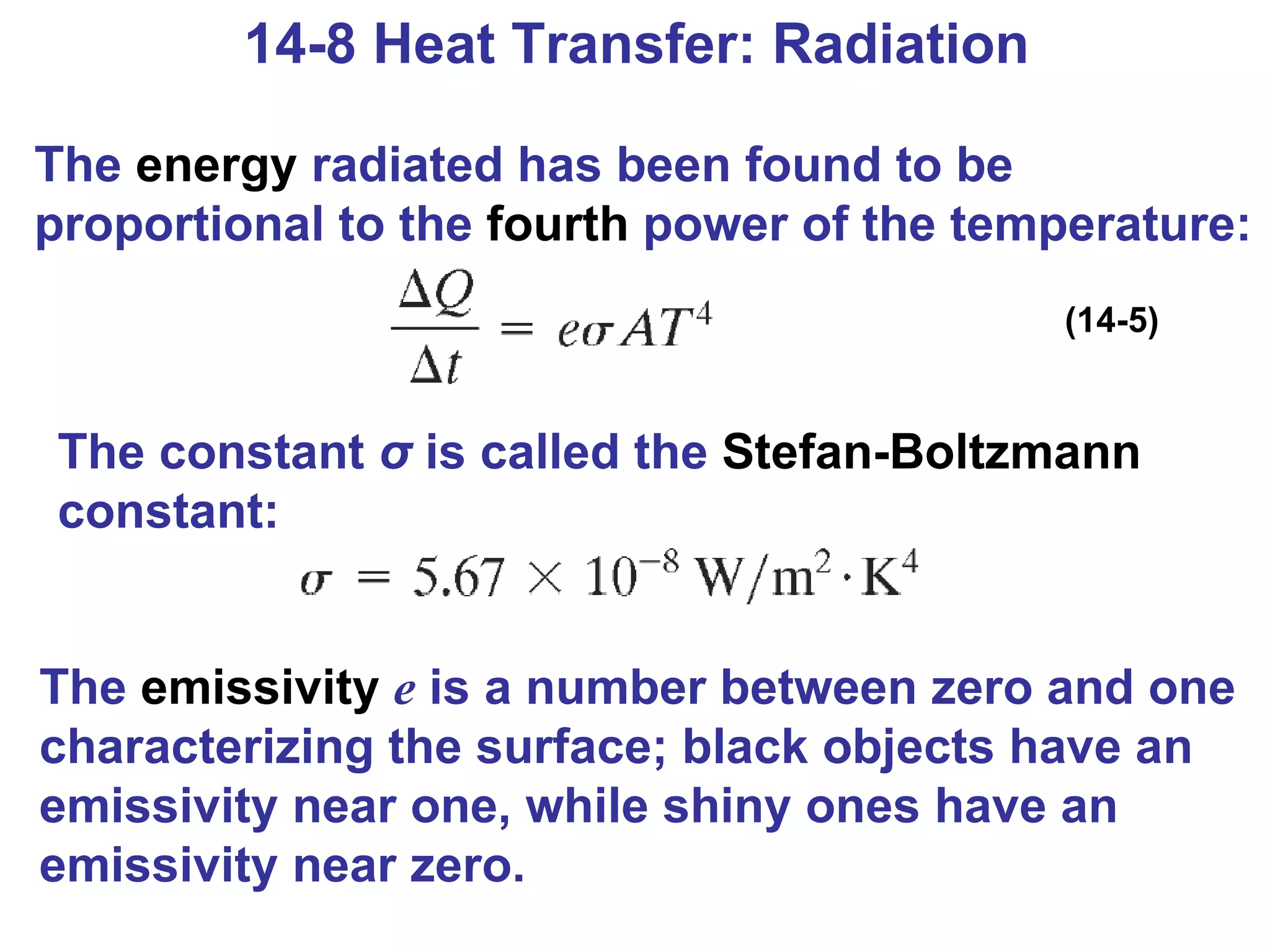
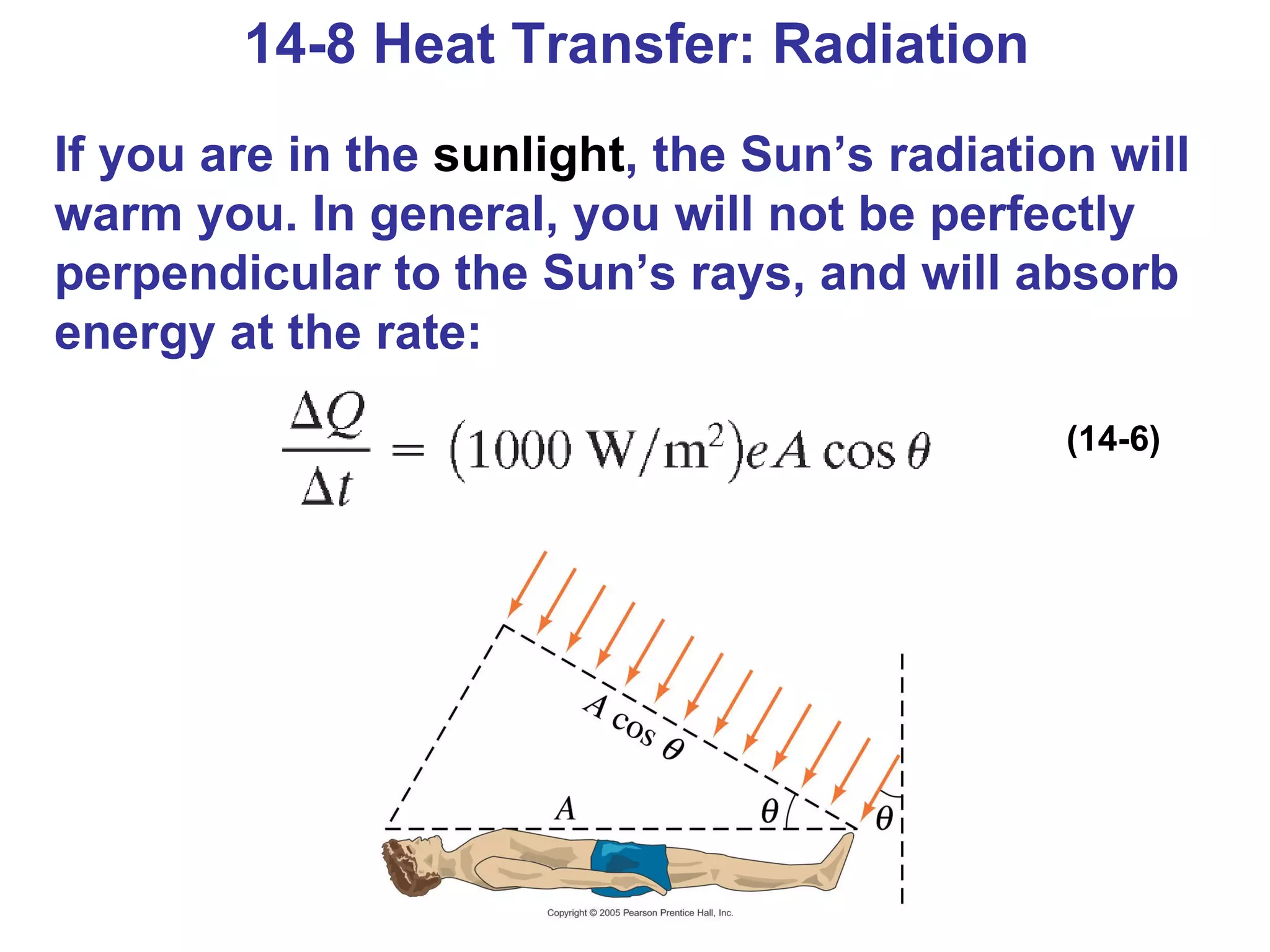
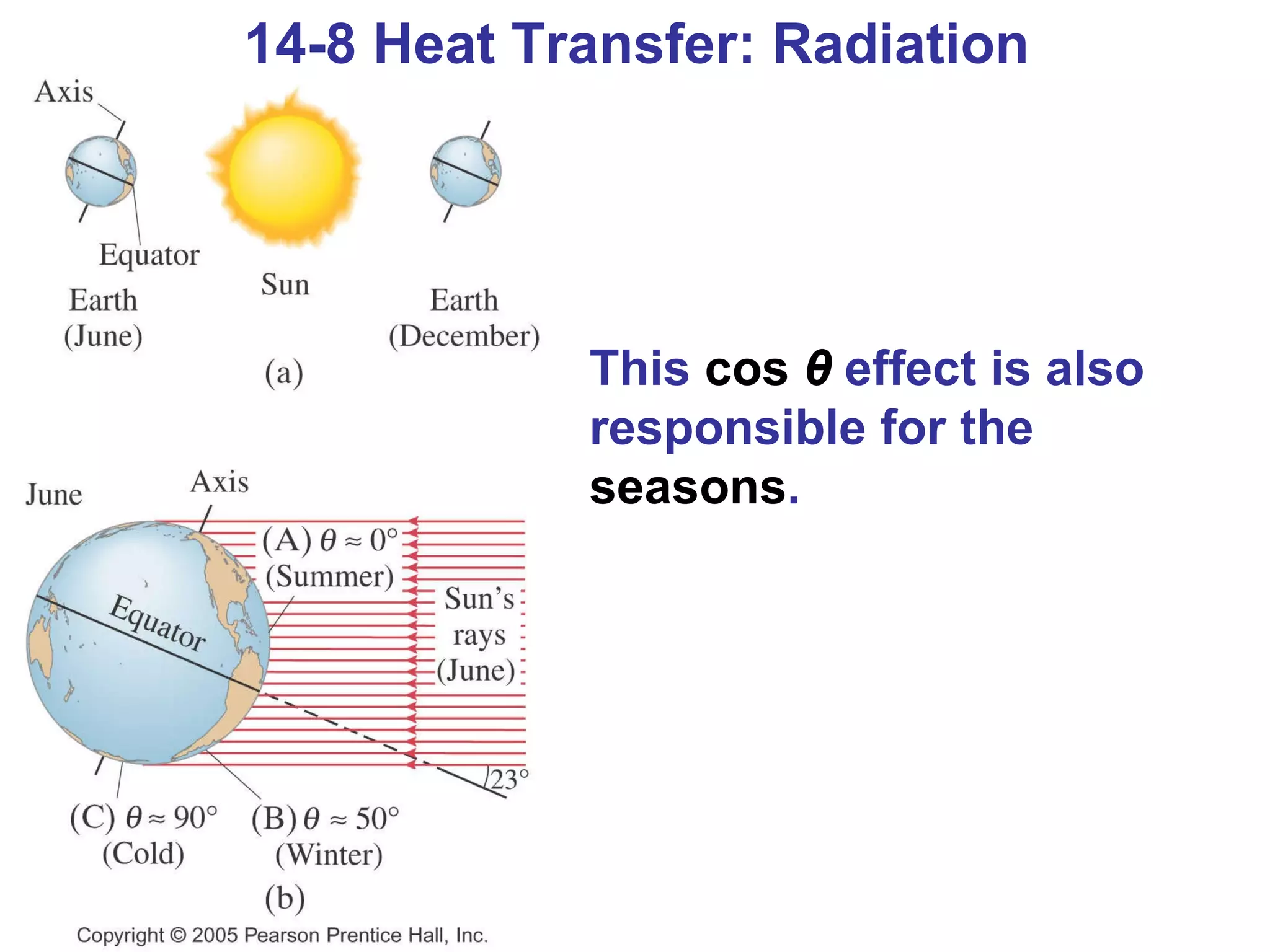
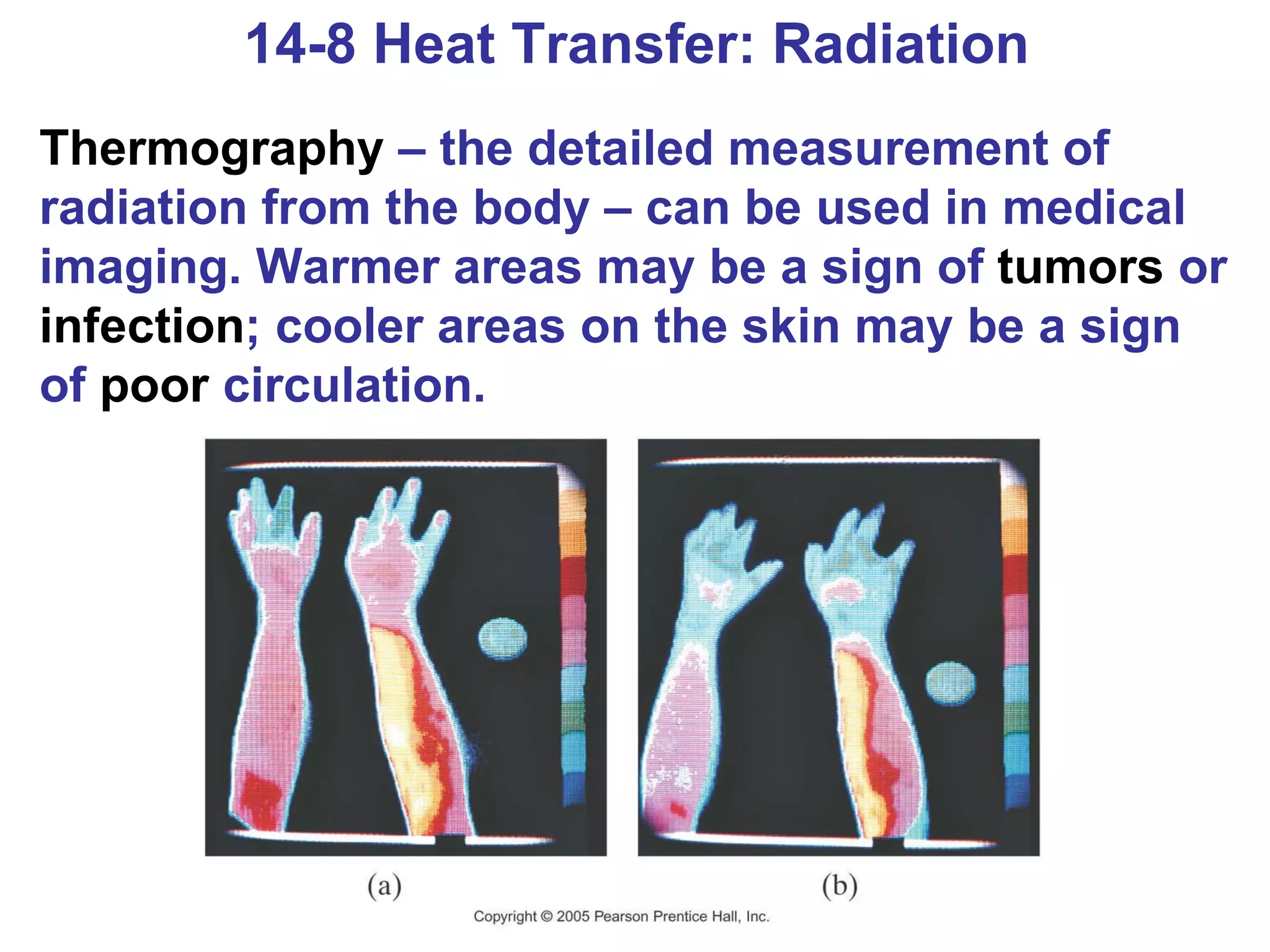
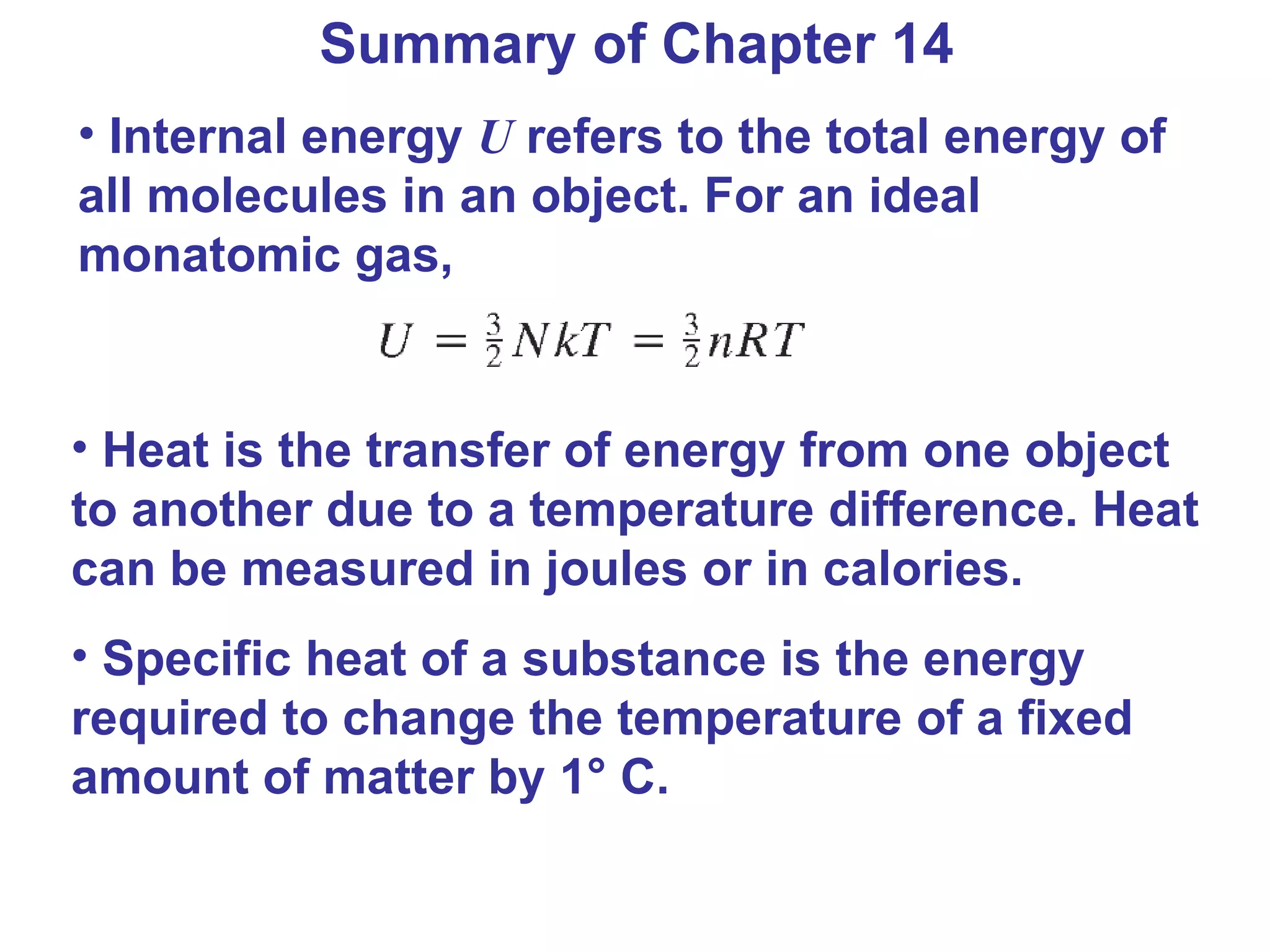
Heat is a form of energy transferred between objects due to a temperature difference. Internal energy refers to the total kinetic energy of all molecules in an object. Specific heat is the energy required to change an object's temperature by 1°C. Phase changes like melting and boiling require energy input in the form of latent heat even as temperature remains constant. Heat transfer occurs through conduction, convection and radiation processes between objects at different temperatures.