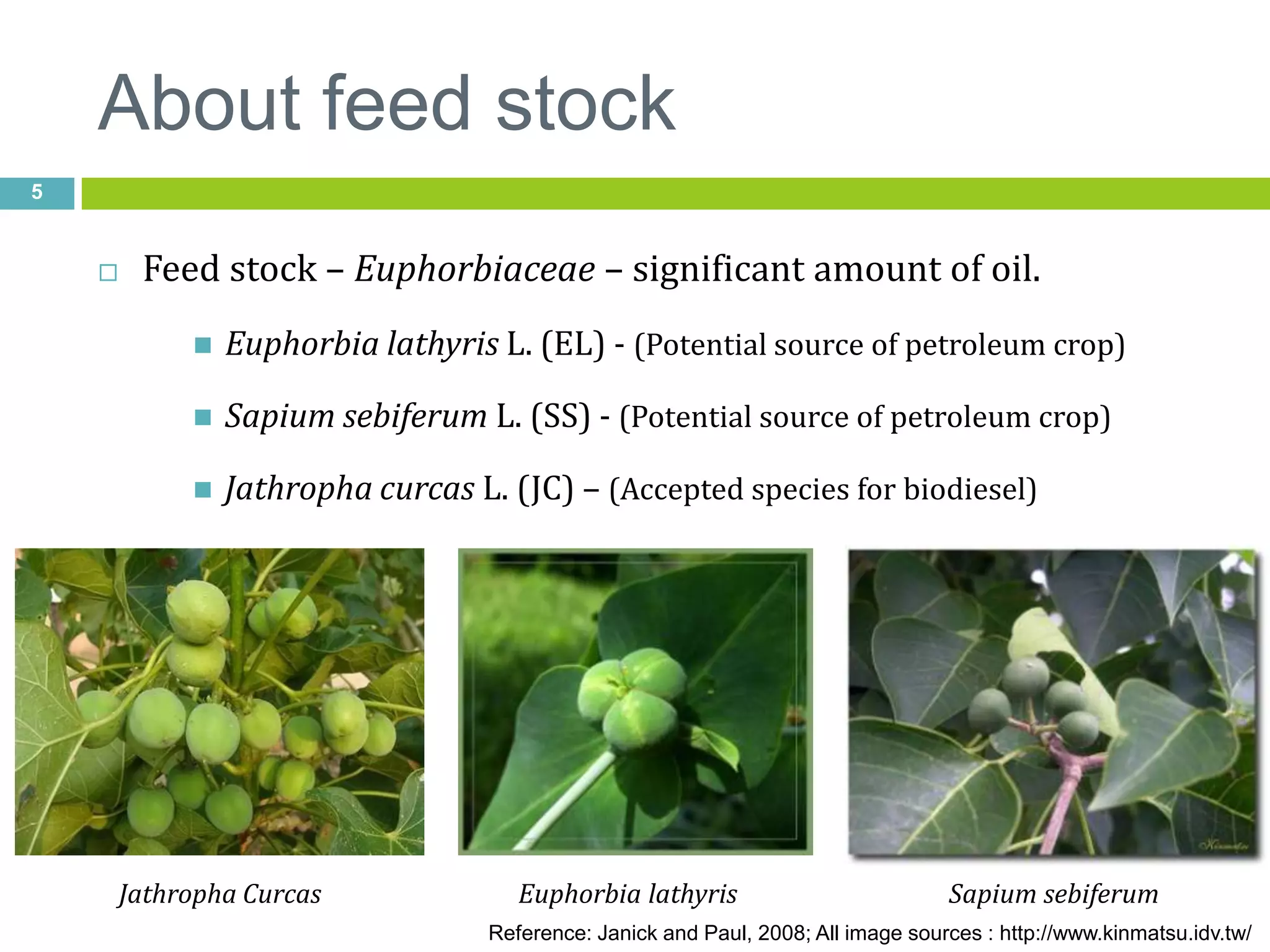
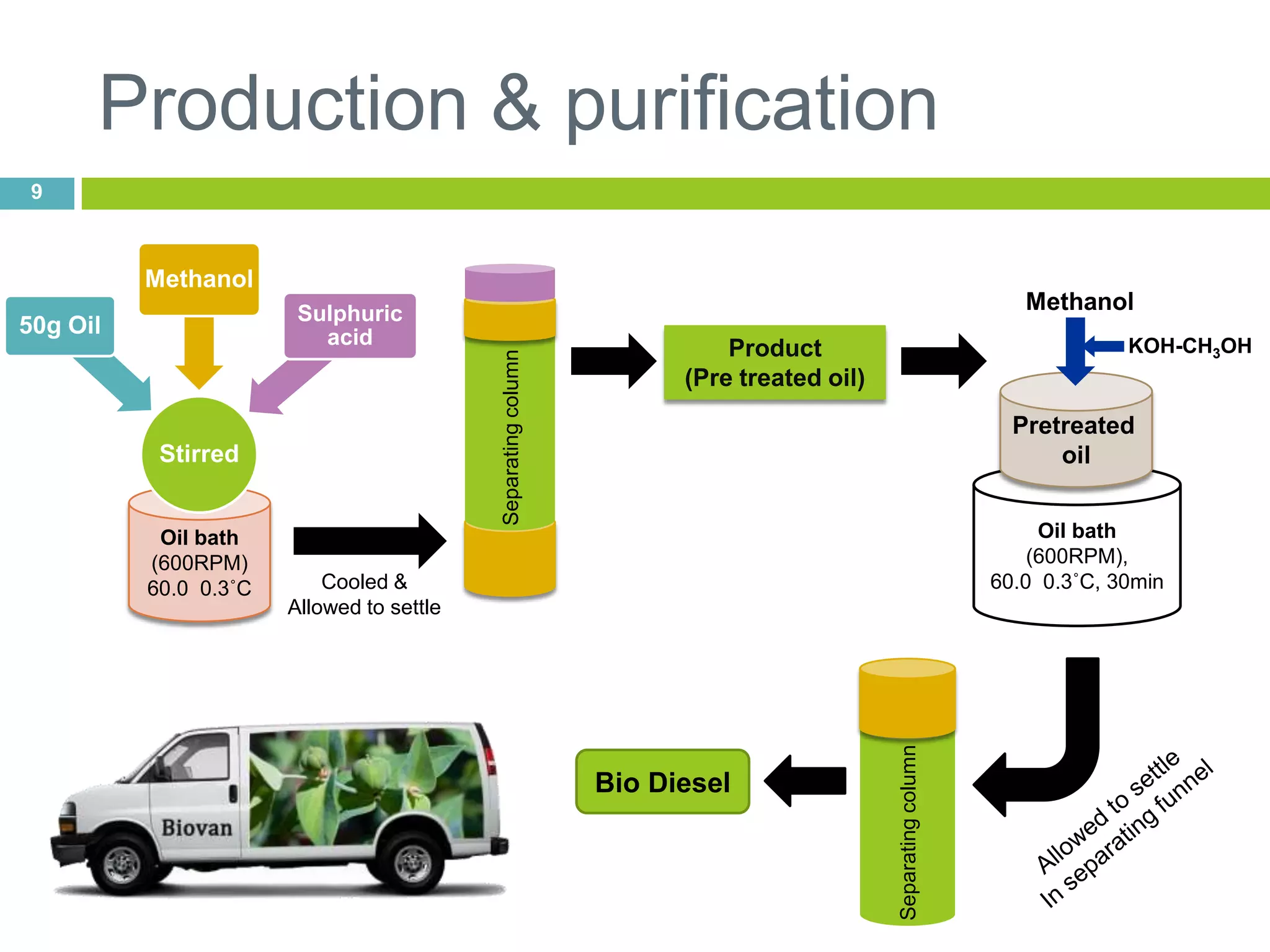
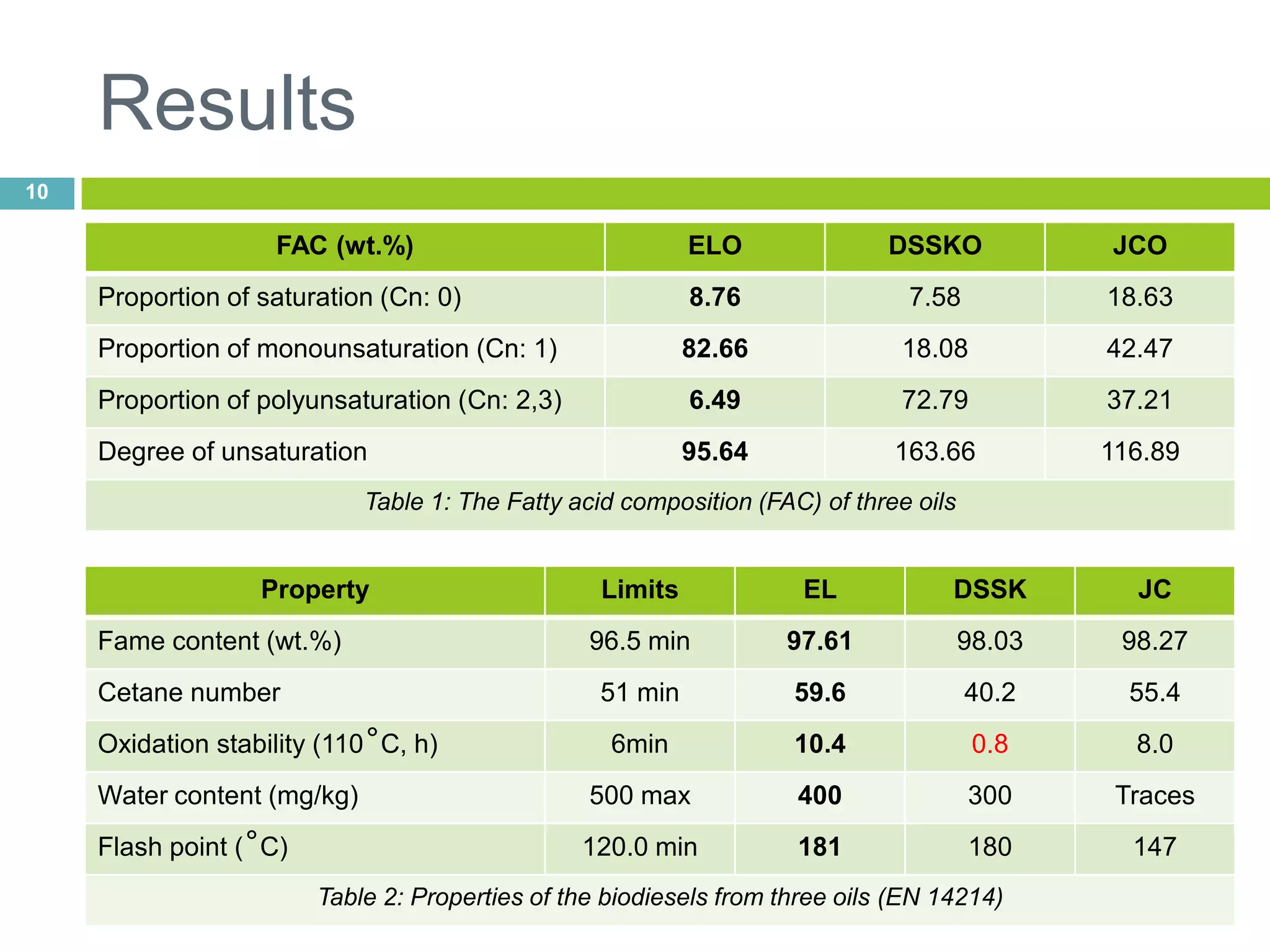
This document discusses the production and properties of biodiesel from non-edible plant oils. It begins by introducing biodiesel as an alternative fuel and its environmental benefits compared to fossil fuels. It then discusses using non-edible oils instead of edible oils to produce biodiesel in order to avoid competition with food resources. The document evaluates various non-edible plant oils for biodiesel production and finds that Euphorbia lathyris oil has superior properties to other candidates tested. It concludes that E. lathyris is a promising biodiesel feedstock.