The document discusses optimization of biodiesel production from sunflower oil using response surface methodology. Key points:
- Biodiesel was produced from sunflower oil through a transesterification process using methanol and a KOH catalyst.
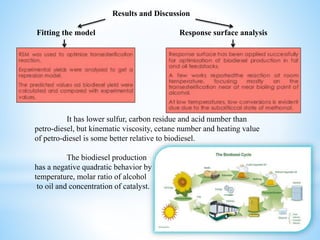
- Experiments were conducted to determine the optimum conditions for transesterification, including temperature, molar ratio of alcohol to oil, and catalyst concentration.
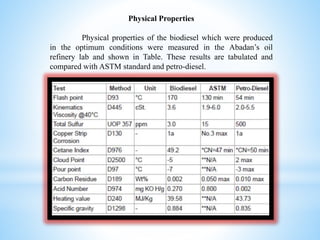
- The results showed that biodiesel produced under optimum conditions met ASTM standards and had environmental benefits over petroleum diesel, though some petroleum diesel properties were better.