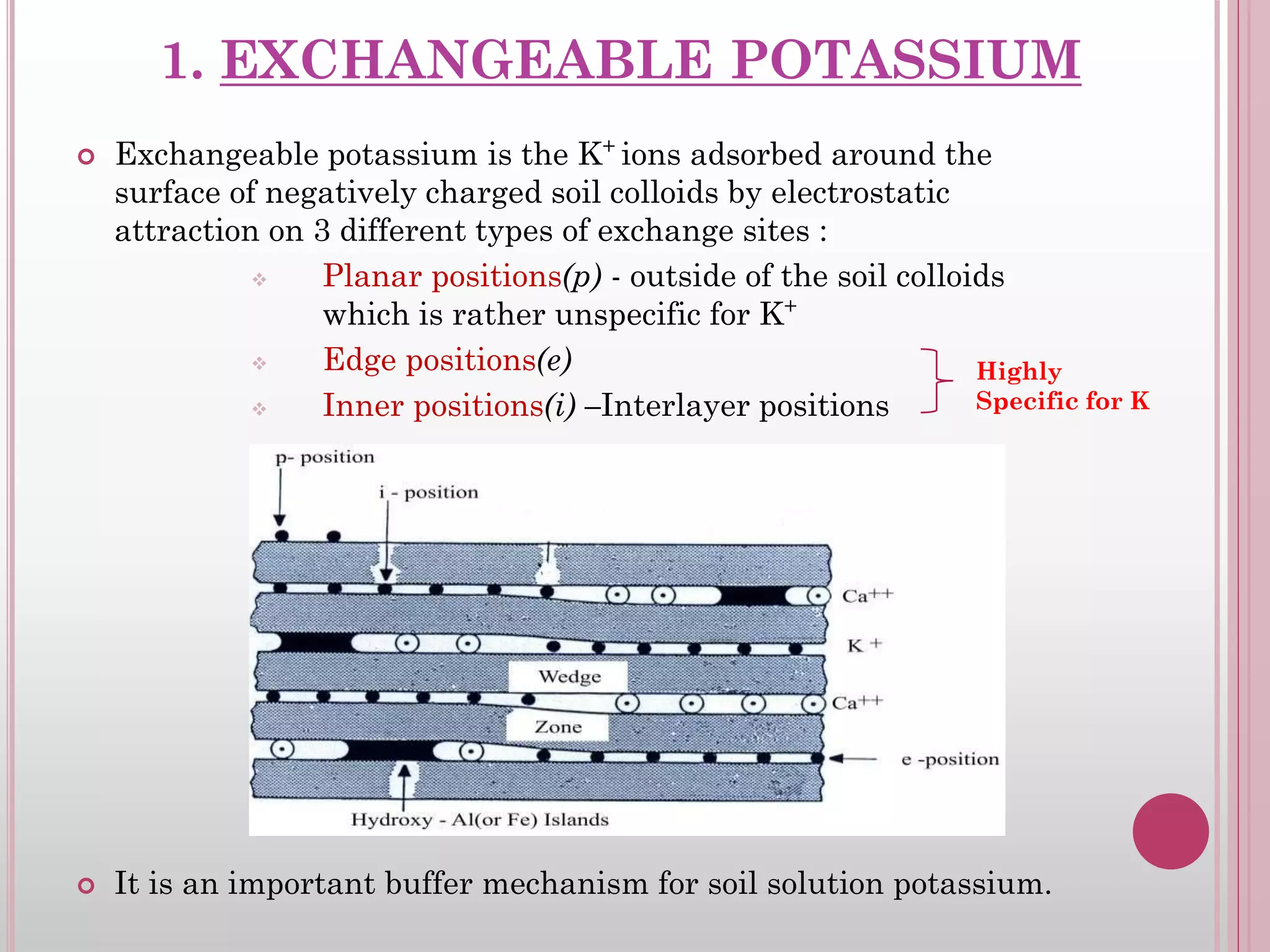
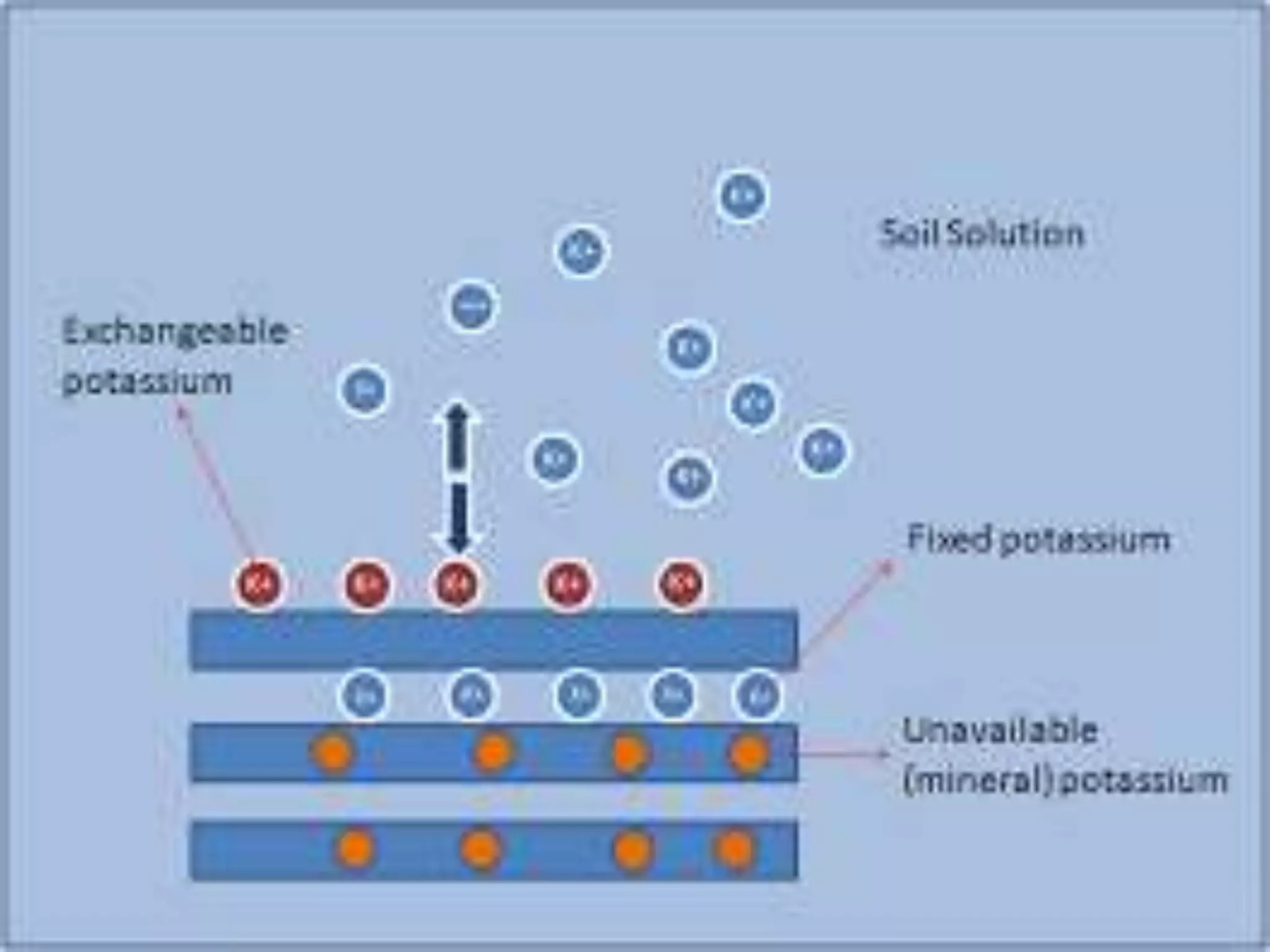
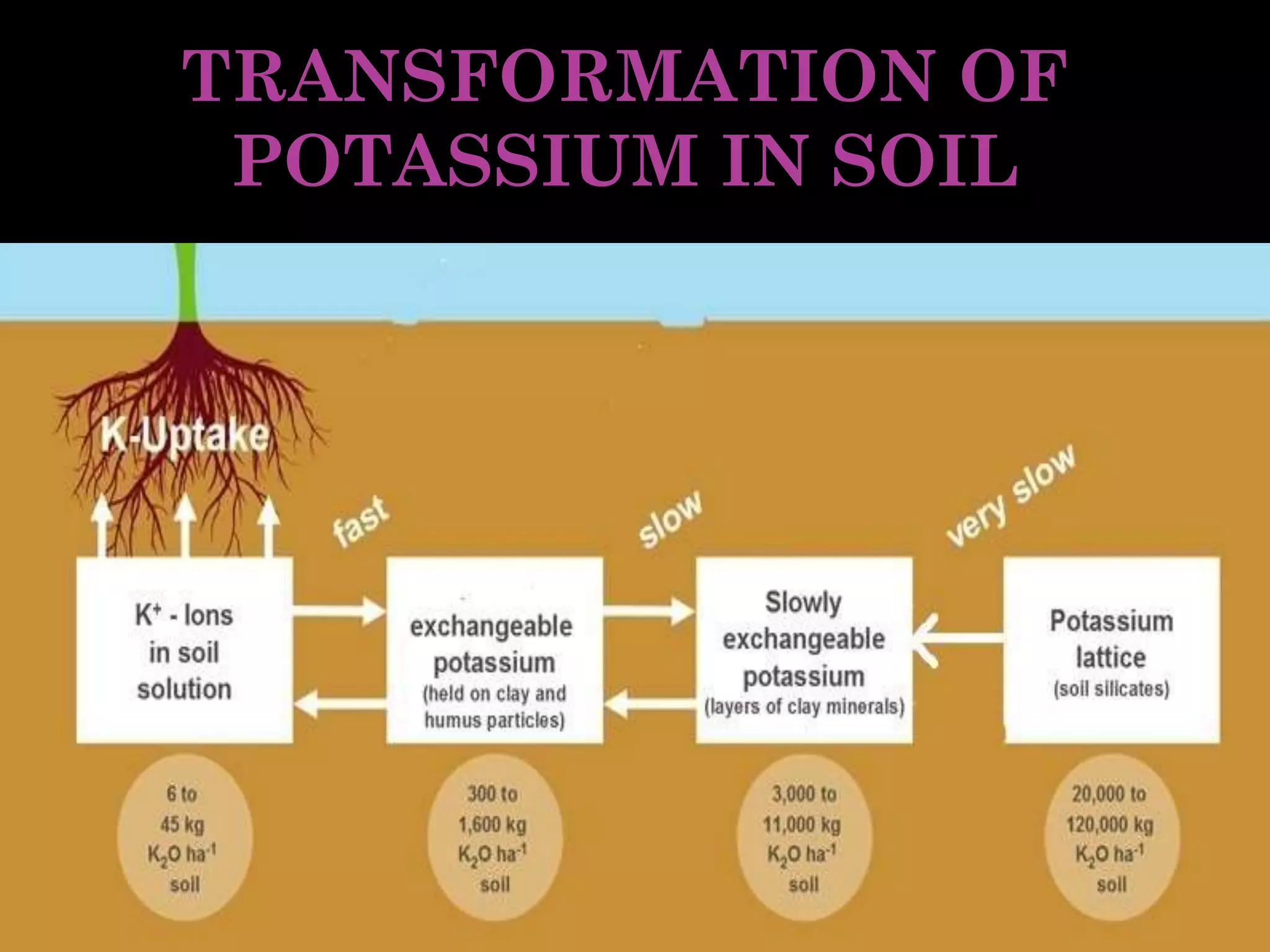
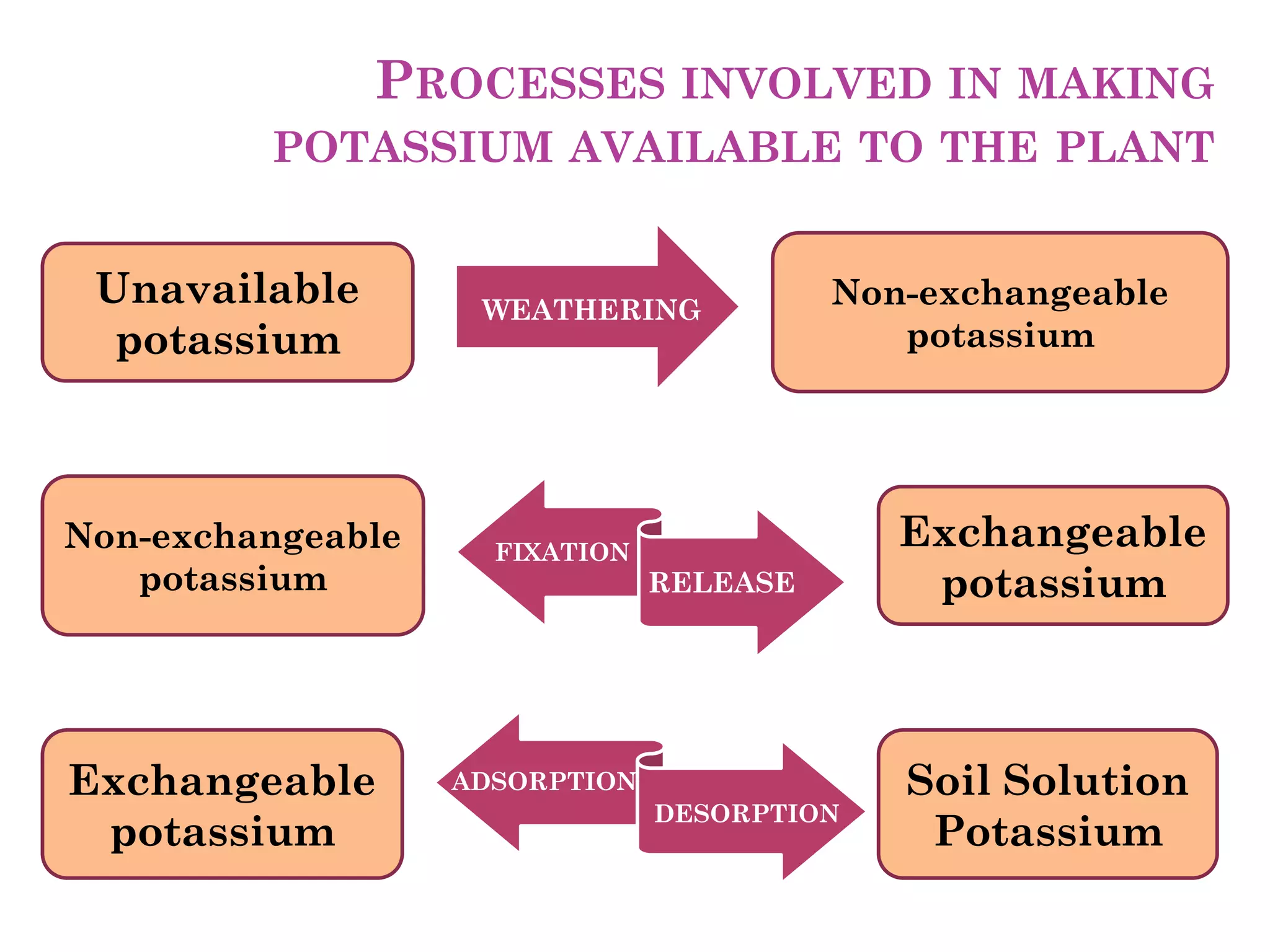
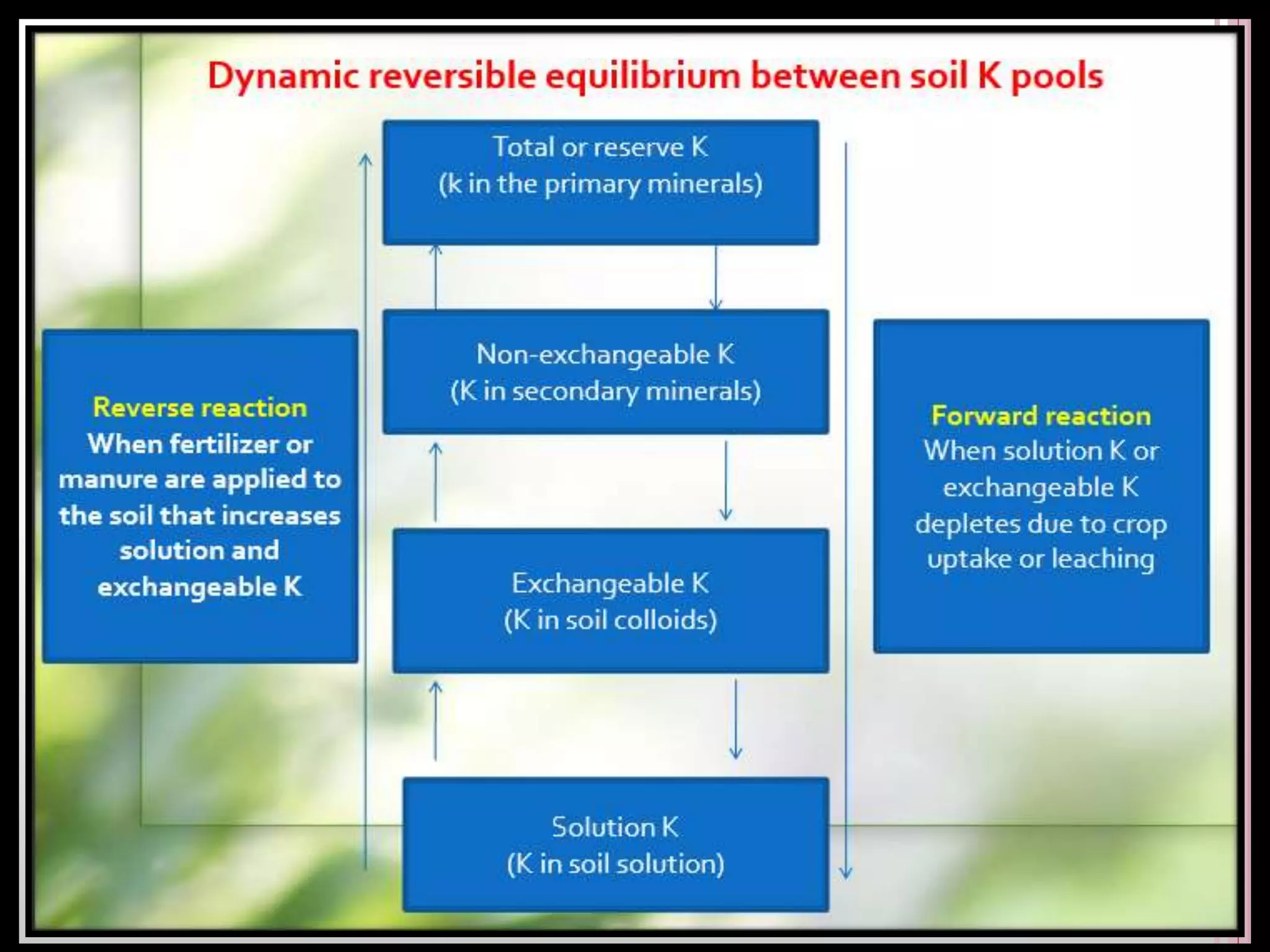
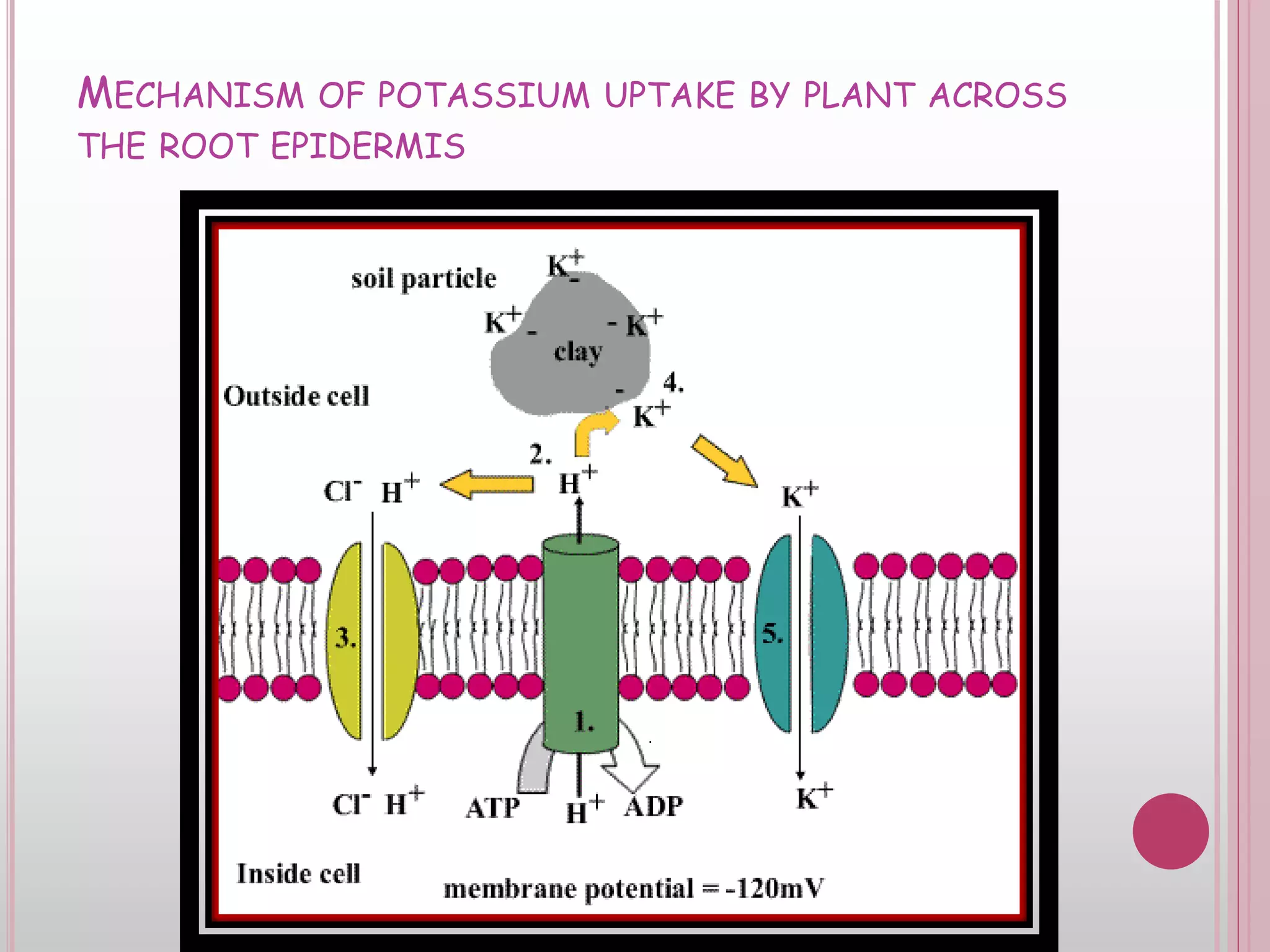
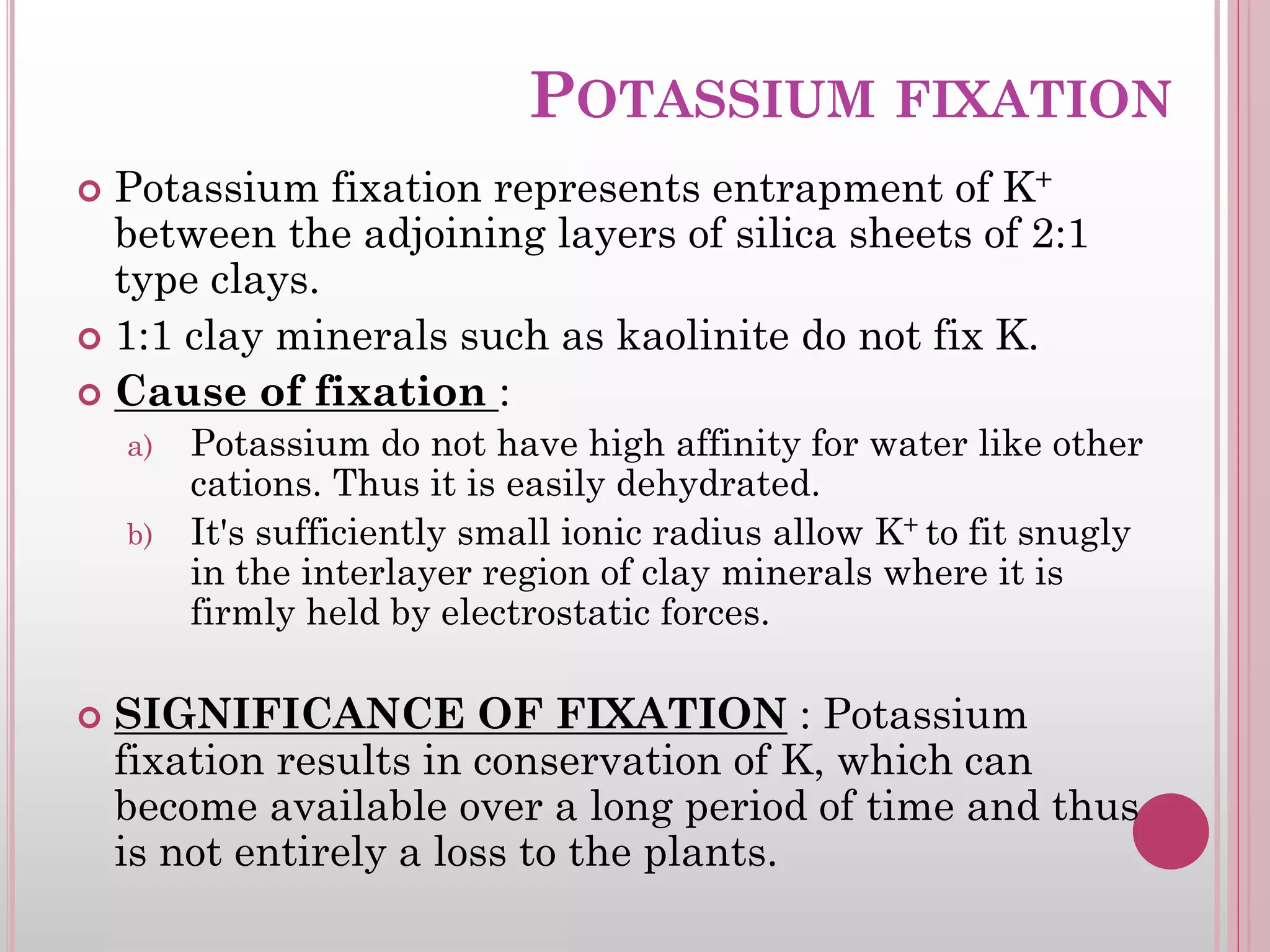
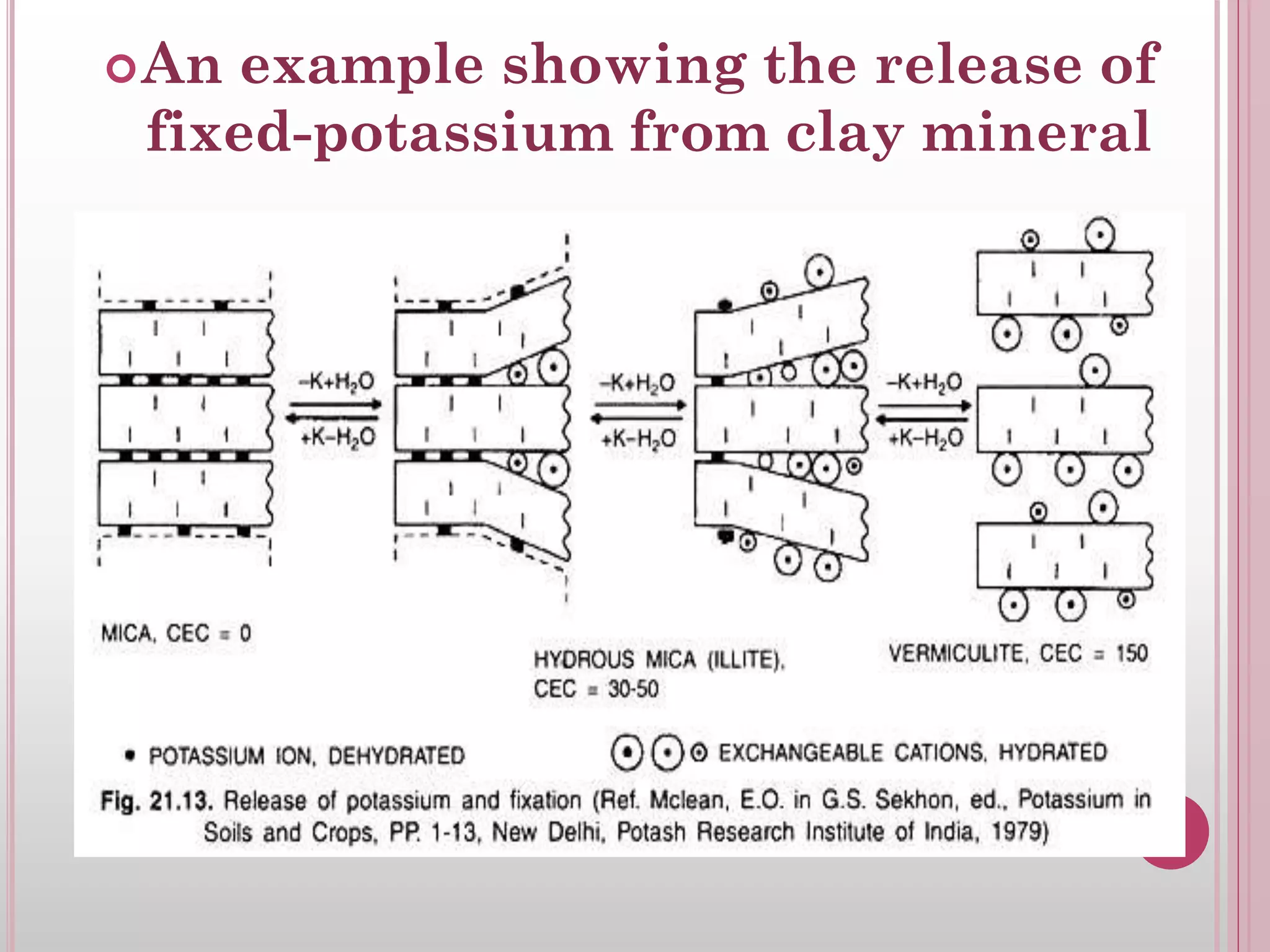
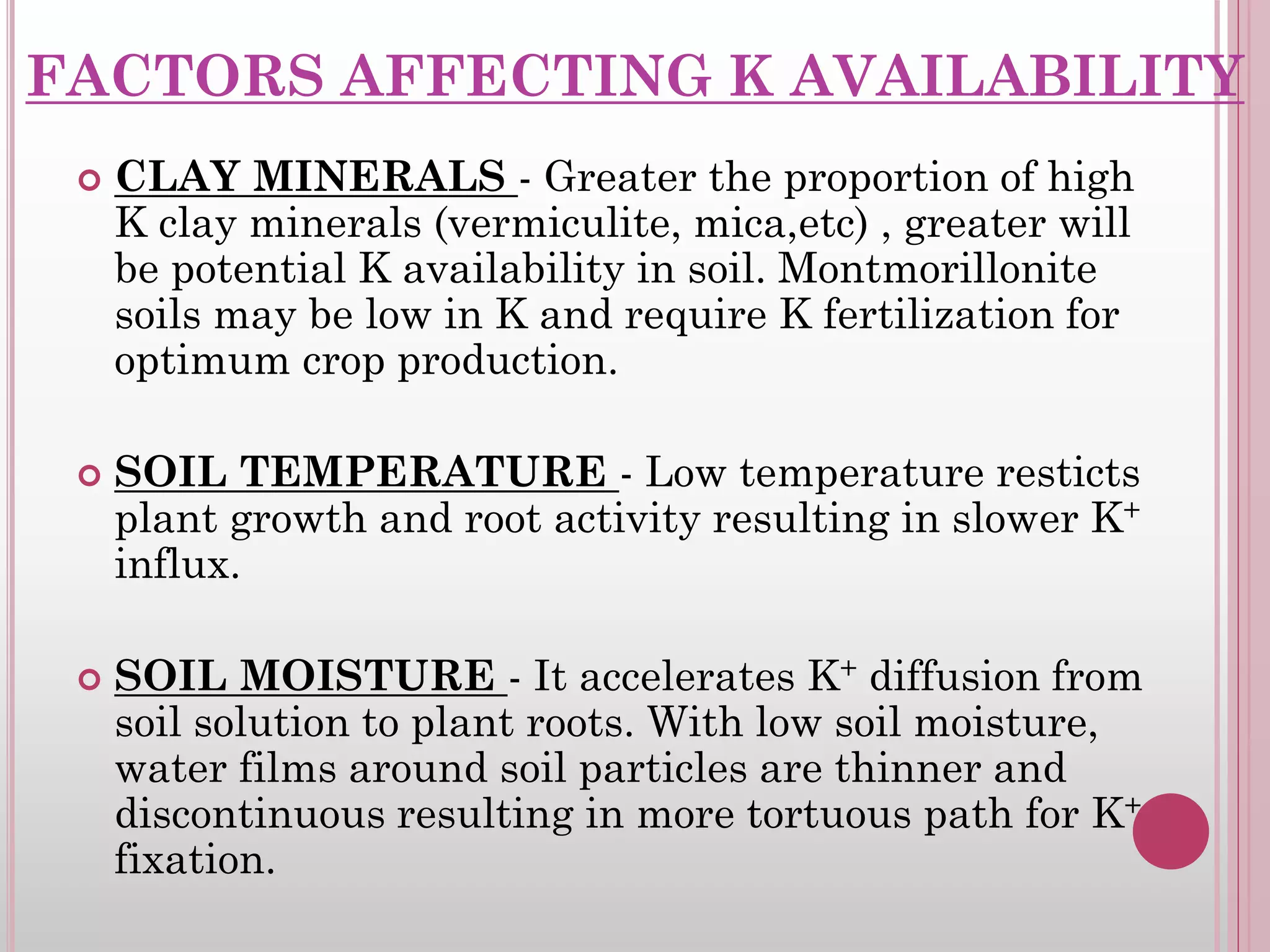
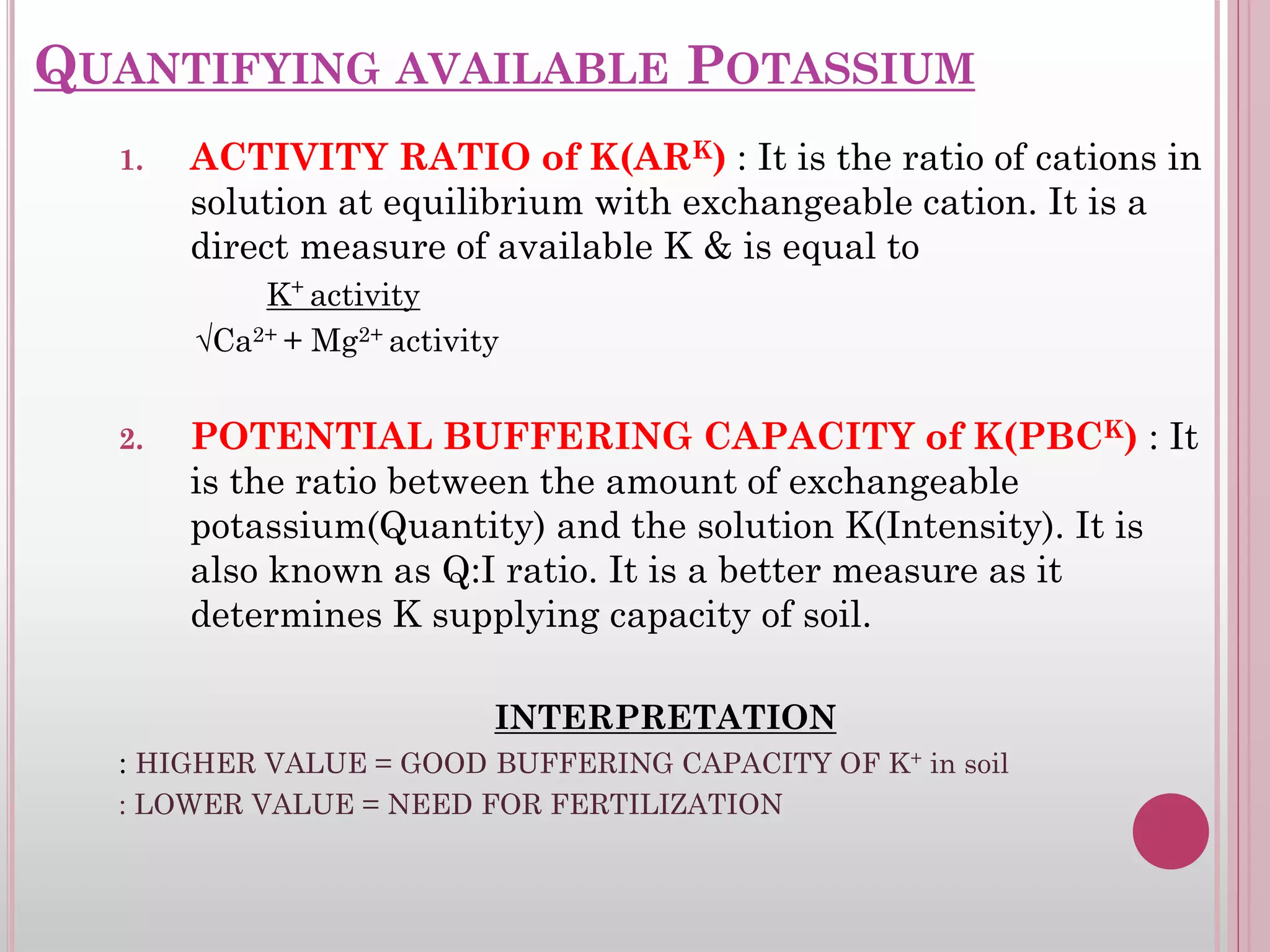
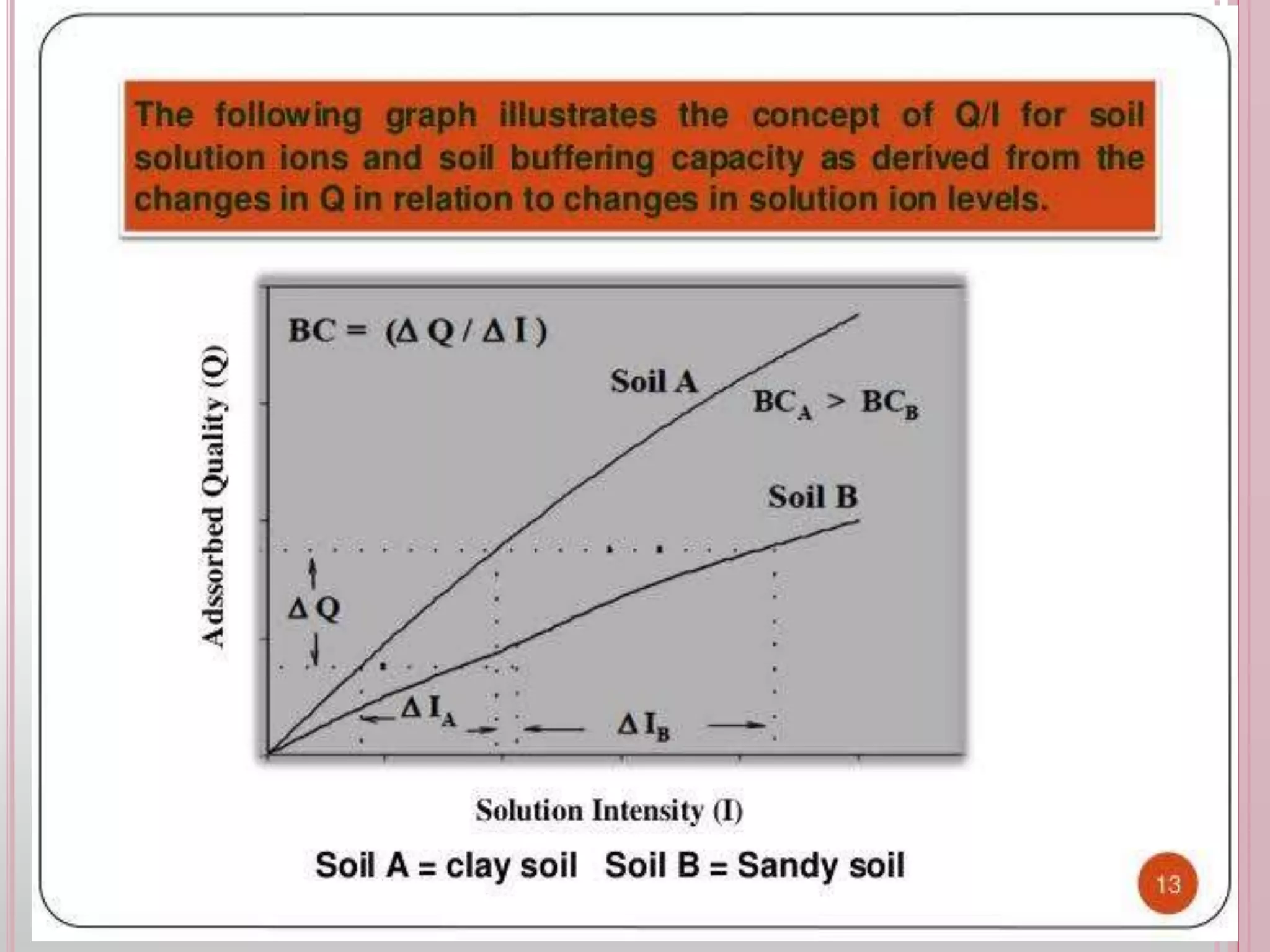
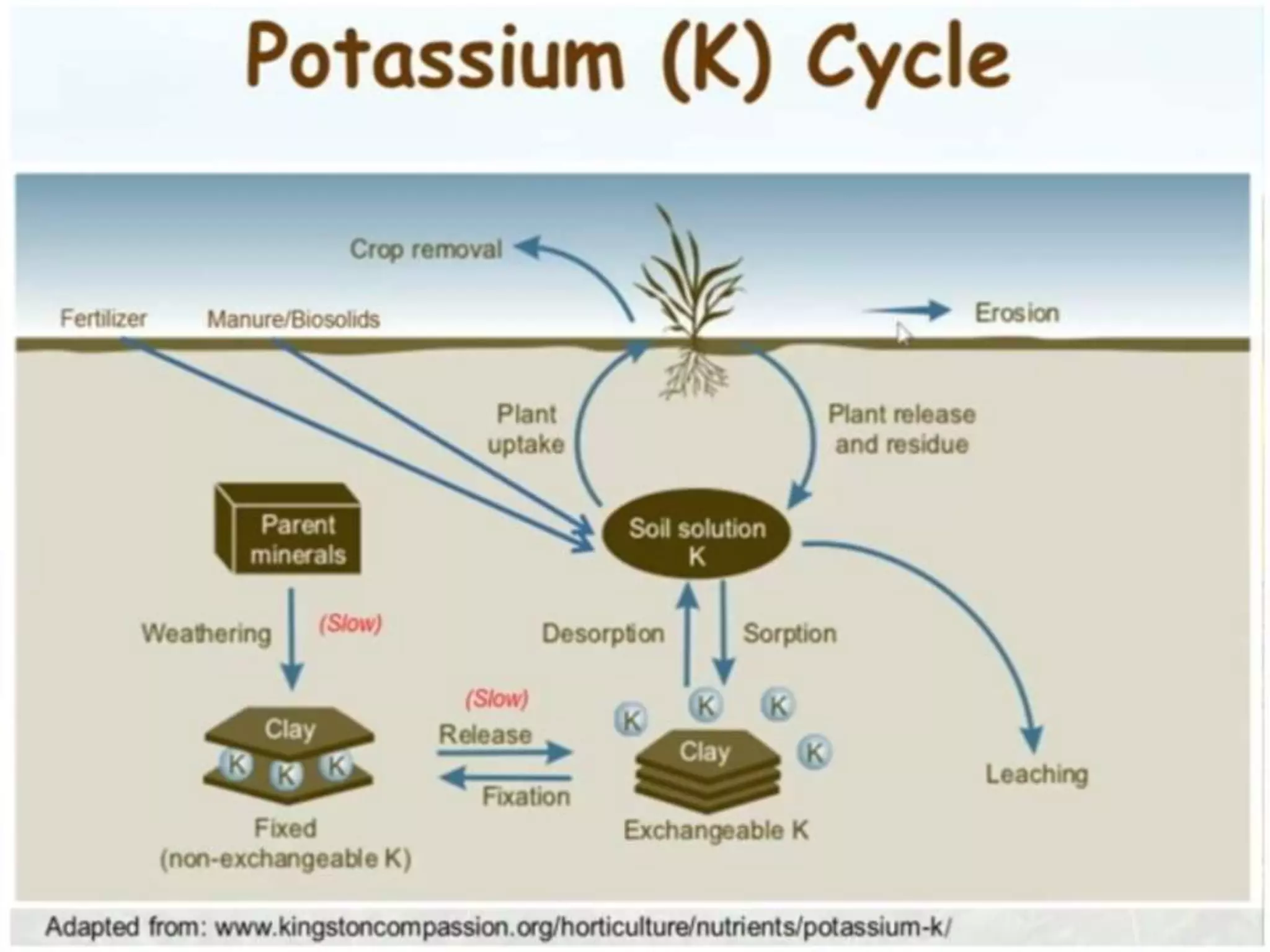
The document is an assignment on potassium's transformation and dynamics in soil for a course on soil fertility and nutrient management. It discusses various forms of potassium, its availability to plants, processes of fertilization, and factors affecting potassium's behavior in soil. Key topics include the potassium cycle, mechanisms of uptake by plants, and the impact of soil conditions on potassium availability.



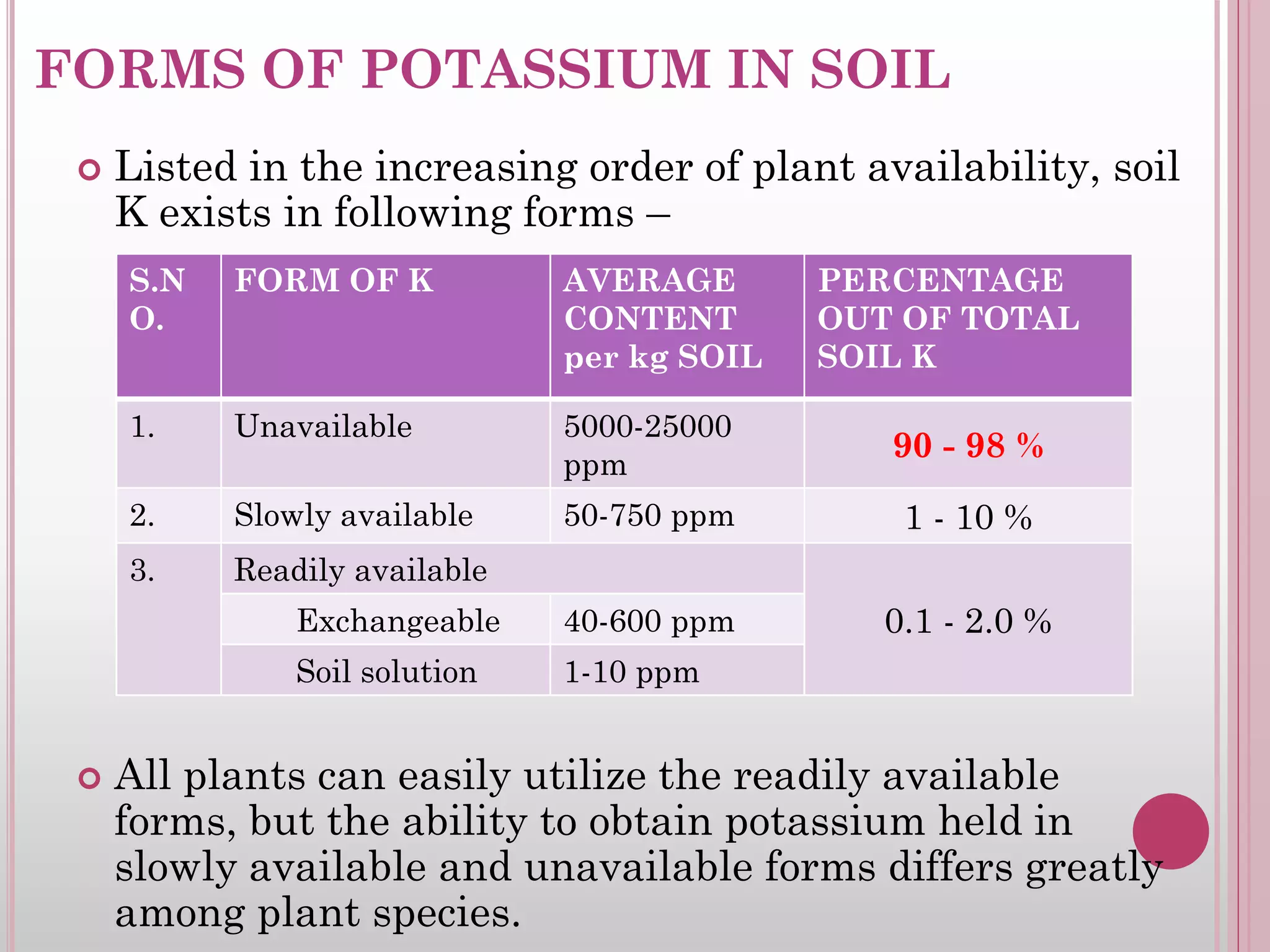
![UNAVAILABLE FORM
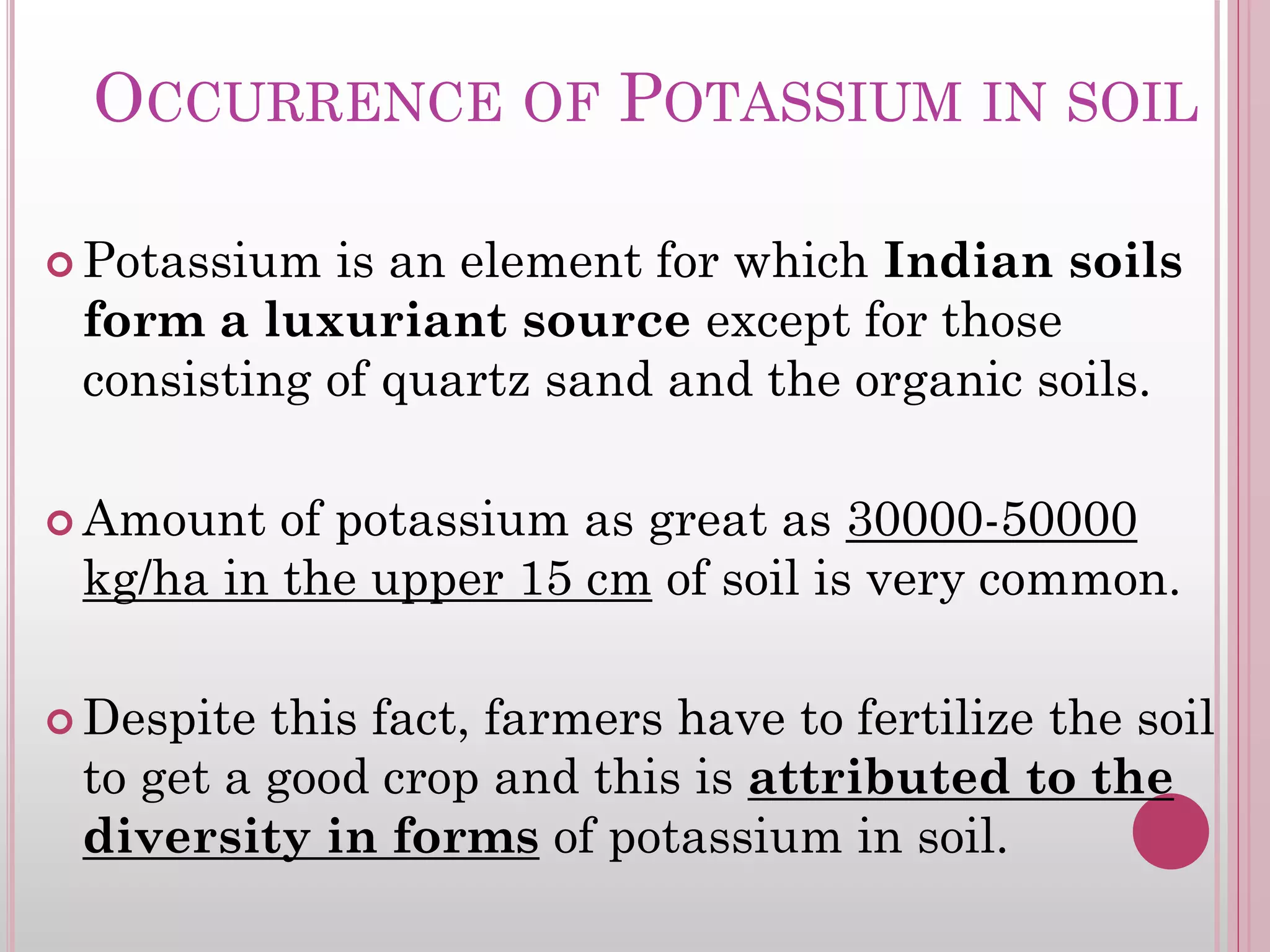
Also known as Mineral K, this is the major natural source of potassium in soil.
It is found on crystal structures of primary minerals like –
Micas – Micas are 2:1 layer silicates where K+ resides mainly between
the layer silicates
• Muscovite : [KAl3Si3O10(OH)2]
• Biotite : [K(Mg,Fe)3 AlSi3O10(OH)2]
• Phlogopite : [KMg2Al2Si3O10(OH)2]
Feldspars – Feldspars are 3-dimensional crystal structure with K
located throughout the mineral lattice. They are the largest natural
potassium reserve.
• Orthoclase
• Microcline : KAlSi3O8
The potassium from this source can be released only through weathering. Thus, they
supply very small quantities of potassium during a given growing season.
Only their cumulative release over a period of years is of some importance which is
enhanced by the solvent action of carbonic acid, stronger organic acid, inorganic
acids, acidic clays and humus.
Order of primary minerals in the ease of weathering is as follows:
Biotite > Muscovite > K-feldspars > Phlogopite
The release of K from these minerals is also geared by the dissolution of the mineral
from the roots of certain plants like elephant grass.](https://image.slidesharecdn.com/transformationsanddynamicsofpotassium-190414053254/75/Transformations-and-dynamics-of-potassium-6-2048.jpg)